Abstract
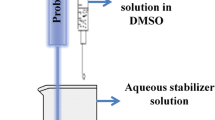
Conventional nanoprecipitation process involves addition of water miscible organic solvent containing drug to an aqueous phase containing hydrophilic surfactants to yield drug nanosuspension. However, nanosuspensions obtained with conventional nanoprecipitation process have very low colloidal stability. The objective of the present investigation was to fabricate drug nanosuspensions with good colloidal stability using a modified nanoprecipitation method. Celecoxib, a hydrophobic anti-inflammatory agent with low oral bioavailability, was used as a model drug for this investigation. The conventional nanoprecipitation method did not result in the nanosizing of the celecoxib. Incorporation of surface active lipophiles such as Labrafil 1944 CS (oleolyl macrogol glycerides) along with hydrophilic surfactants during nanoprecipitation process could successfully nanosize the celecoxib. The particle size of the nanosuspensions was influenced by the various parameters of the nanoprecipitation process and also by the concentration of the lipophilic stabilizer. The celecoxib nanosuspension was characterized by transmission electron microscopy, differential scanning calorimetry, and X-ray diffraction. Saturation solubility of celecoxib was dramatically improved in pH 1.2 buffer when formulated as nanosuspensions. The celecoxib nanosuspesnsion showed significantly higher in vitro dissolution rate and in vivo anti-inflammatory activity as compared to that of celecoxib-marketed formulation.






Similar content being viewed by others
References
Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–40.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62:3–16.
Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96.
Möschwitzer J, Achleitner G, Pomper H, Müller RH. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58:615–9.
Liu Y, Huang L, Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm. 2010;7:863–9.
Kobierski S, Ofori-Kwakye K, Müller RH, Keck CM. Resveratrol nanosuspensions for dermal application—production, characterization, and physical stability. Pharmazie. 2009;64:741–7.
Pardeike J, Müller RH. Dermal and ocular safety of the new phospholipase A2 inhibitors PX-18 and PX-13 formulated as drug nanosuspension. J Biomed Nanotechnol. 2009;5:437–44.
Kassem MA, Abdel Rahman AA, Ghorab MM, Ahmed MB, Khalil RM. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340:126–33.
Chiang PC, Alsup JW, Lai Y, Hu Y, Heyde BR, Tung D. Evaluation of aerosol delivery of nanosuspension for pre-clinical pulmonary drug delivery. Nanoscale Res Lett. 2009;4:254–61.
Shubar HM, Lachenmaier S, Heimesaat MM, Lohman U, Mauludin R, Mueller RH, et al. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood–brain barriers. J Drug Target. 2011;19:114–24.
Sjöström B, Kronberg B, Carlfors J. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. I: influence of emulsification and surfactant concentration. J Pharm Sci. 1993;82:579–83.
Sjöström B, Bergenståhl B, Kronberg B. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. II: influence of the emulsifier, the solvent, and the drug substance. J Pharm Sci. 1993;82:584–9.
Trotta M, Gallarate M, Pattarino F, Morel S. Emulsions containing partially water-miscible solvents for the preparation of drug nanosuspensions. J Control Release. 2001;76:119–28.
Dolenc A, Kristl J, Baumgartner S, Planinsek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12.
Xia D, Quan P, Piao H, Piao H, Sun S, Yin Y, et al. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40:325–34.
Shekunov BY, Chattopadhyay P, Seitzinger J, Huff R. Nanoparticles of poorly water-soluble drugs prepared by supercritical fluid extraction of emulsions. Pharm Res. 2006;23:196–204.
Trotta M, Gallarate M, Carlotti ME, Morel S. Preparation of griseofulvin nanoparticles from water-dilutable microemulsions. Int J Pharm. 2003;254:235–42.
Margulis-Goshen K, Kesselman E, Danino D, Magdassi S. Formation of celecoxib nanoparticles from volatile microemulsions. Int J Pharm. 2010;393:230–7.
Ali HS, York P, Blagden N. Preparation of hydrocortisone nanosuspension through a bottom–up nanoprecipitation technique using microfluidic reactors. Int J Pharm. 2009;375:107–13.
Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004;27:1993–9.
Nagarsenker MS, Joshi MS. Celecoxib-cyclodextrin systems: characterization and evaluation of in vitro and in vivo advantage. Drug Dev Ind Pharm. 2005;31:169–78.
Liu Y, Sun C, Hao Y, Jiang T, Zheng L, Wang S. Mechanism of dissolution enhancement and bioavailability of poorly water soluble celecoxib by preparing stable amorphous nanoparticles. J Pharm Pharm Sci. 2010;13:589–606.
Margulis-Goshen K, Weitman M, Major DT, Magdassi S. Inhibition of crystallization and growth of celecoxib nanoparticles formed from volatile microemulsions. J Pharm Sci. 2011;100:4390–400.
Joshi M, Patravale V. Nanostructured lipid carrier (NLC) based gel of celecoxib. Int J Pharm. 2008;346:124–32.
Higuchi WI, Misra J. Physical degradation of emulsions via the molecular diffusion route and the possible prevention thereof. J Pharm Sci. 1962;51:459–66.
Kabalnov AS, Pertzov AV, Shchukin ED. Ostwald ripening in two-component disperse phase systems: application to emulsion stability. Colloids Surf. 1987;24:19–32.
Lindfors L, Skantze P, Skantze U, Rasmusson M, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 1. Inhibition of Ostwald ripening. Langmuir. 2006;22:906–10.
Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, Kim DD, et al. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72.
Yoo HS. Preparation of biodegradable polymeric hollow microspheres using O/O/W emulsion stabilized by Labrafil. Colloids Surf B: Biointerfaces. 2006;52:47–51.
Wang S, Sun M, Ping Q. Enhancing effect of Labrafac Lipophile WL 1349 on oral bioavailability of hydroxysafflor yellow A in rats. Int J Pharm. 2008;358:198–204.
Jain AS, Date AA, Nagarsenker MS. Design, characterization and evaluation of anti-epileptic activity of nanoprecipitating preconcnetrate of carbamazepine. Drug Deliv Lett. 2013;3:61–9.
Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci. 2008;35:183–92.
Date AA, Desai N, Dixit R, Nagarsenker MS. Self-nanoemulsifying drug delivery systems (SNEDDS): formulation insights, applications and advances. Nanomedicine (Lond). 2010;5:1596–616.
Acknowledgments
Authors are thankful to CAD Pharma Inc, Aurobindo Pharmaceuticals Pvt. Ltd., Gangwal Chemicals Pvt. Ltd., Gattefosse India Ltd., BASF India Ltd., Sasol GmBH, and Phospholipid GmBH, Germany, for providing gift samples of drug and excipients. Authors are also thankful to Tata Institute of Fundamental Research, Mumbai, India, for proving XRD, SEM, and DSC facilities and to Jaslok Hospital for TEM facilities. Anju Malakani is thankful to AICTE for junior research fellowship.
Conflict of interest
The authors have no conflict of interest in any part of this investigation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Malkani, A., Date, A.A. & Hegde, D. Celecoxib nanosuspension: single-step fabrication using a modified nanoprecipitation method and in vivo evaluation. Drug Deliv. and Transl. Res. 4, 365–376 (2014). https://doi.org/10.1007/s13346-014-0201-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-014-0201-3