Abstract
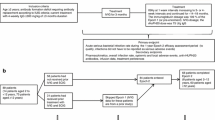
Many patients with primary immunodeficiency disease (PIDD) require lifelong immunoglobulin (Ig) replacement therapy. Home-based subcutaneous (SC) infusion provides advantages to patients with PIDD compared to hospital-based intravenous infusion. One limitation of current practice with SCIg infusion is the need for small-volume infusions at multiple injection sites on a frequent basis. A method was developed for large-volume SC infusion that uses preinfusion of recombinant human hyaluronidase (rHuPH20) to facilitate fluid dispersion. Miniature swine was used as a preclinical model to assess the effects of rHuPH20-facilitated infusions, of a single monthly dose, on fluid dispersion, infusion-related pressure, swelling, induration, and tissue damage. Preinfusion of vehicle (control) or rHuPH20 (75 U/g Ig) was performed simultaneously on contralateral abdominal sites on each animal, followed by infusion of 300 mL 10 % Ig (30 g) at each site. Compared to control infusions, rHuPH20 significantly reduced infusion pressure and induration (p < 0.05) and accelerated postinfusion Ig dispersion. Histological evaluation of infusion site tissue showed moderate to severe swelling for the control. Swelling after rHuPH20-facilitated infusion was mild on day 1 and had completely resolved shortly thereafter. Laser Doppler imaging of control infusion sites revealed local cutaneous hypoperfusion during Ig infusion, which was reduced almost 7-fold (p < 0.05) with the use of rHuPH20. These results demonstrate that rHuPH20-facilitated Ig infusion is associated with improved dispersion of Ig, resulting in reduced tissue pressure, induration, and reduced risk of tissue damage from mechanical trauma or local ischemia, thus enabling SC administration of large volumes of Ig at a single site.







Similar content being viewed by others
References
Rezaei N, Hedayat M, Aghamohammadi A, Nichols KE. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J Allergy Clin Immunol. 2011;127(6):1329–41.
Moise A, Nedelcu FD, Toader MA, Sora SM, Tica A, Ferastraoaru DE, Constantinescu I. Primary immunodeficiencies of the B lymphocyte. J Med Life. 2010;3(1):60–3.
Gardulf A. Immunoglobulin treatment for primary antibody deficiencies. BioDrugs. 2007;21:105–16.
Kittner JM, Grimbacher B, Wulff W, Jäger B, Schmidt RE. Patients’ attitude to subcutaneous immunoglobulin substitution as home therapy. J Clin Immunol. 2006;26(4):400–5.
Gardulf A, Nicolay U. Replacement Ig therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2006;6(6):434–42.
Misbah S, Sturzenegger MH, Borte M, Shapiro RS, Wasserman RL, Berger M, Ochs HD. Subcutaneous immunoglobulin: opportunities and outlook. Clin Exp Immunol. 2009;158 Suppl 1:51–9.
Gustafson R, Gardulf A, Hansen S, Leibl H, Engl W, Lindén M, Müller A, Hammarstrӧm L. Rapid subcutaneous immunoglobulin administration every second week results in high and stable serum immunoglobulin G levels in patients with primary antibody deficiencies. Clin Exp Immunol. 2008;152:274–9.
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous Ig Study Group. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73.
Borte M, Bernatowska E, Ochs HD, Roifman CM, Vivaglobin Study Group. Efficacy and safety of home-based subcutaneous immunoglobulin replacement therapy in paediatric patients with primary immunodeficiencies. Clin Exp Immunol. 2011;164(3):357–64.
Gardulf A, Nicolay U, Math D, Asensio O, Bernatowska E, Böck A, Costa-Carvalho BT, Granert C, Haag S, Hernández D, Kiessling P, Kus J, Matamoros N, Niehues T, Schmidt S, Schulze I, Borte M. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous Ig self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42.
Nicolay U, Kiessling P, Berger M, Gupta S, Yel L, Roifman CM, Gardulf A, Eichmann F, Haag S, Massion C, Ochs HD. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous Ig self-infusions at home. J Clin Immunol. 2006;26(1):65–72.
Hizentra Package Insert. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM203150.pdf.
Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Contr Release. 2006;114(2):230–41.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Exp Opin Drug Deliv. 2007;4:427–40.
NCT00814320 at ClinicalTrials.gov. Gammagard Liquid and rHuPH20 in PID. Available at http://www.clinicaltrials.gov/ct2/show/NCT00814320?term=NCT00814320&rank=1.
Melamed I, Wasserman RL, Stein M, Rubinstein A, McCoy B, Engl W, Leibl H, Gelmont D, Grossman WJ, Schiff RI, and the IGSC, 10 % rHuPH20 Study Group. Tolerability of human immunoglobulin 10 % (Ig) administered subcutaneously (SC) or facilitated with recombinant human hyaluronidase (rHuPH20) in patients with primary immunodeficiency disease (PIDD). Presented at the Annual Meeting of the Clinical Immunology Society, May 19–22, 2011. Abstract #28.
Wasserman RL, Melamed I, Stein M, Rubinstein A, McCoy B, Engl W, Leibl H, Gelmont D, Grossman WJ, Schiff RI, and the IGSC, 10 % rHuPH20 Study Group. Pharmacokinetics (PK) of human immunoglobulin 10 % (Ig) administered intravenously (IV), subcutaneously (SC) or facilitated with recombinant human hyaluronidase (rHuPH20) in patients with primary immunodeficiency disease (PIDD). Presented at the Annual Meeting of the Clinical Immunology Society, May 19–22, 2011. Abstract #53.
Svendsen O. The minipig in toxicology. Exp Toxicol Pathol. 2006;57:335–9.
van der Laan JW, Brightwell J, McAnulty P, Ratky J, Stark C, Steering Group of the RETHINK Project. Regulatory acceptability of the minipig in the development of pharmaceuticals, chemicals and other products. J Pharm Toxicol Methods. 2010;62(3):184–95.
Mahl JA, Vogel BE, Court M, Kolopp M, Roman D, Nogués V. The minipig in dermatotoxicology: methods and challenges. Exp Toxicol Pathol. 2006;57(5–6):341–5.
Nunoya T, Shibuya K, Saitoh T, Yazawa H, Nakamura K, Baba Y, Hirai T. Use of miniature pig for biomedical research, with reference to toxicologic studies. J Tox Pathol. 2007;20:125–32.
Golla S, Madihally S, Robinson Jr RL, Gasem KA. Quantitative structure–property relationship modeling of skin sensitization: a quantitative prediction. Toxicol Vitro. 2009;23(3):454–65.
Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1–2):157–71.
Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9.
Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7.
Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;285(4):779–802.
Chouksey A, Duff K, Wasserbauer N, Berger M. Subcutaneous immunoglobulin-g replacement therapy with preparations currently available in the United States for intravenous or intramuscular use: reasons and regimens. Allergy Asthma Clin Immunol. 2005;1(3):120–30.
Singh S. Acute compartment syndrome. Curr Orthop. 2004;18:468–76.
Steinberg BD. Evaluation of limb compartments with increased interstitial pressure. An improved noninvasive method for determining quantitative hardness. J Biomech. 2005;38(8):1629–35.
Mars M, Hadley GP. Raised intracompartmental pressure and compartment syndrome. Injury. 1998;29(6):403–11.
Kalns J, Cox J, Baskin J, Santos A, Odland R, Recura Jr S. Threshold model for extremity compartment syndrome in swine. J Surg Res. 2011;167(1):13–9.
Leu AJ, Leu HJ, Franzeck UK, Bollinger A. Microvascular changes in chronic venous insufficiency—a review. Cardiovasc Surg. 1995;3(3):237–45.
Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran JV, Huygen FJ, Bennett GJ, Coderre TJ. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol Pain. 2008;4:49.
Uhlmann D, Armann B, Gaebel G, Ludwig S, Hess J, Pietsch UC, Escher E, Fieldler M, Tannapfel A, Hauss J, Witzigmann H. Endothelin A receptor blockade reduces hepatic ischemia/reperfusion injury after warm ischemia in a pig model. J Gastrointest Surg. 2003;7(3):331–9.
Reddy NP, Cochran GV. Interstitial fluid flow as a factor in decubitus ulcer formation. J Biomech. 1981;14(12):879–81.
Acknowledgments
The authors would like to thank Ghia Bacani for formulation support, James Skipper and Adrian Radi for assistance with the preclinical studies, Rebecca Symons for histology support, Dr. Michael J. Tomlinson for histopathological assessment, Dr. Ping Jiang for statistical analysis of the histopathological assessment, and Drs. Daniel C. Maneval, David A. Gold, Curtis B. Thompson, and H. Michael Shepard for critical review of the manuscript. Medical writing support, funded by Baxter BioScience, was provided by Roland Tacke, Candace Lundin, and Josh Collis of Gardiner-Caldwell Communications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, D.W., Jadin, L., Nekoroski, T. et al. Recombinant human hyaluronidase PH20 (rHuPH20) facilitates subcutaneous infusions of large volumes of immunoglobulin in a swine model. Drug Deliv. and Transl. Res. 2, 254–264 (2012). https://doi.org/10.1007/s13346-012-0065-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-012-0065-3