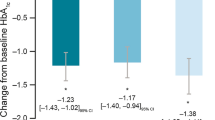Abstract
Aims
We aimed to identify diabetes-related predictors associated with achieving glycosylated hemoglobin (HbA1c) <7.0 % by using data from the Add-on Lantus® to Oral Hypoglycemic Agents (ALOHA) study, a 24-week observational study of Japanese type 2 diabetes patients.
Materials and methods
Among insulin-naïve patients (n = 3515), those who achieved HbA1c <7.0 % at the final visit were categorized as achievers and others as nonachievers. Relationships between baseline factors and achievement of HbA1c <7.0 % were examined by univariate and multivariate analysis.
Results
Of 3,515 patients, 545 (15.5 %) achieved HbA1c <7.0 % at 24 weeks (achievers), whereas 2,970 (84.5 %) did not achieve the target level (nonachievers). Patients with diabetes duration <1 year were more likely to achieve HbA1c <7.0 % than those with diabetes duration ≥5 years (27 % for <1; 17 % for ≥1, <5; and 15 % for ≥5: P < 0.05 for the trend). The HbA1c <7.0 % responder rate was higher in those with baseline HbA1c <8.5 % than in the other baseline HbA1c categories (28 %; P < 0.001 vs. all other higher categories). Patients with retinopathy were less likely to achieve HbA1c <7.0 % (P < 0.001). These three factors also showed significant associations in the multivariate logistic regression model.
Conclusions
This study revealed shorter diabetes duration (<1 year), lower HbA1c (<8.5 %), and no retinopathy at baseline to be significantly associated with a higher rate of achieving the target HbA1c in insulin-naïve patients started on therapy with insulin glargine plus oral antidiabetic drugs. These results suggest that earlier initiation of insulin glargine will raise the likelihood of type 2 diabetes patients achieving optimal glycemic control (HbA1c <7.0 %).





Similar content being viewed by others
References
Japan Diabetes Society. Treatment guide for diabetes 2010. Tokyo: Bunkodo Co., Ltd.; 2010.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–9.
Wake N, Hisashige A, Katayama T, Kishikawa H, Ohkubo Y, Sakai M, Araki E, Shichiri M. Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study. Diabetes Res Clin Pract. 2000;48:201–10.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–7.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Kennedy L, Herman WH, Strange P, Harris A. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care. 2006;29:1–8.
Ohtani T, Ito T. Safety and effectiveness of BOT (Basal supported Oral Therapy) using insulin glargine in Japanese patients with type 2 diabetes—results from postmarketing surveillance of insulin glargine (ALOHA study). Shinyaku to Rinsho (J New Rem Clin). 2011;60:458–75.
Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in Real Life: ALOHA study subanalysis. Diabetes Technol Ther. 2012. doi:10.1089/dia.2011.0220.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int. 2012;3:8–10.
Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–2.
R Development Core Team. R: a language and environment for statistical computing. In: R Foundation for Statistical Computing; 2011.
Sharplin P, Gordon J, Peters JR, Tetlow AP, Longman AJ, McEwan P. Switching from premixed insulin to glargine-based insulin regimen improves glycaemic control in patients with type 1 or type 2 diabetes: a retrospective primary-care-based analysis. Cardiovasc Diabetol. 2009;8:9.
Gordon J, Pockett RD, Tetlow AP, McEwan P, Home PD. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64:1609–18.
Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract. 2006;73:35–40.
Tamaki M, Shimizu T, Kanazawa A, Fujitani Y, Watada H, Kawamori R, Hirose T. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract. 2008;81:e1–3.
Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
Acknowledgments
The authors thank all physicians at the 987 hospitals and clinics participating in the ALOHA study. This study was sponsored by sanofi-aventis K.K.
Conflict of interest
TK received an honorarium for a lecture from sanofi-aventis K.K.; TK and MO received advisory board fees as publication committee members; TO works for sanofi-aventis K.K.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kadowaki, T., Ohtani, T. & Odawara, M. Baseline predictive factors for glycemic control in Japanese type 2 diabetes patients treated with insulin glargine plus oral antidiabetic drugs: ALOHA study subanalysis. Diabetol Int 4, 16–22 (2013). https://doi.org/10.1007/s13340-012-0087-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-012-0087-6