Abstract
Background and Objectives
Indole-3-carbinol (I3C) is reported to have neuroprotective properties in an animal model of ischemic stroke. However, the pharmacokinetics of I3C in stroke animals are unknown. Furthermore, the most effective method of I3C delivery for the treatment of stroke has yet to be determined. Therefore, the objective of this study was to evaluate pharmacokinetics and pharmacodynamics of I3C to discover the most effective delivery route for protecting the brain from ischemic injury.
Methods
With oral and intravenous administration, the pharmacokinetics and pharmacodynamics of I3C in sham and middle cerebral artery occluded (MCAO) rats were investigated.
Results
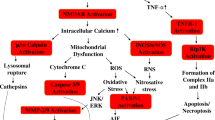
I3C administration in sham and MCAO rats did not alter the pharmacokinetic parameters such as maximum plasma concentration (Cmax), time to reach Cmax, half-life, area under the curve, mean residential time, volume of distribution, clearance, bioavailability, and tissue distribution. A higher amount of diindolylmethane (DIM) was observed with oral administration of I3C compared to intravenous administration in the plasma (5 fold), brain (4 fold), and cerebrospinal fluid (CSF) (2–3 fold). Orally delivered I3C significantly reduced neurological deficits, brain infarction (20%), blood-brain barrier leakage (15 μg/g), and brain water content (75%) in MCAO rats compared to intravenous administration of I3C.
Conclusions
I3C pharmacokinetic parameters were similar in sham and MCAO rats, but I3C and DIM penetration in the brain and CSF was significantly higher in MCAO rats than in sham animals, and I3C oral intake significantly reduced MCAO-induced neurological impairments. Consequently, compared to intravenous treatment, I3C oral delivery is more effective in treating ischemic stroke.
Graphical abstract









Similar content being viewed by others
References
Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4(9):1201–15.
Exon J, et al. Effects of indole-3-carbinol on immune responses, aberrant crypt foci, and colonic crypt cell proliferation in rats. J Toxicol Environ Health Part A. 2001;62(7):561–73.
El-Naga RN, Mahran YF. Indole-3-carbinol protects against cisplatin-induced acute nephrotoxicity: role of calcitonin gene-related peptide and insulin-like growth factor-1. Sci Rep. 2016;6:29857.
Ramakrishna, K. and S. Krishnamurthy, Indole-3-carbinol ameliorated the isoproterenol-induced myocardial infarction via multimodal mechanisms in Wistar rats. Nat Prod Res. 2022:36: 1–6.
Garikapaty V, et al. Anti-carcinogenic and anti-metastatic properties of indole-3-carbinol in prostate cancer. Oncol Rep. 2005;13(1):89–93.
El-Naga RN, Ahmed HI, Al Haleem ENA. Effects of indole-3-carbinol on clonidine-induced neurotoxicity in rats: Impact on oxidative stress, inflammation, apoptosis and monoamine levels. Neurotoxicology. 2014;44:48–57.
Ping J, et al. Therapeutic effect of indole-3-carbinol on pig serum-induced hepatic fibrosis in rats. Yao Xue Xue Bao Acta Pharm Sin. 2011;46(8):915–21.
Souli E, et al. Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem Toxicol. 2008;46(3):863–70.
Paliwal P, Chauhan G, Gautam D, Dash D, Patne SCU, Krishnamurthy S. Indole-3-carbinol improves neurobehavioral symptoms in a cerebral ischemic stroke model. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(6):613–25.
Bansal S, Sangha KS, Khatri P. Drug treatment of acute ischemic stroke. Am J Cardiovasc Drugs. 2013;13(1):57–69.
Fisher M, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50.
Park MK, Rhee Y-H, Lee H-J, Lee E-O, Kim K-H, Park M-J, Jeon B-H, Shim B-S, Jung C-H, Choi S-H. Antiplatelet and antithrombotic activity of indole-3-carbinol in vitro and in vivo. Phytother Res Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2008;22(1):58–64.
De Kruif C, et al. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80(3):303–15.
Shertzer HG, Senft AP. The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug Metab Drug Interact. 2000;17(1–4):159–88.
Moussata J, Wang Z, Wang J. Development and validation of an HPLC method for the simultaneous quantification of indole-3-carbinol acetate, indole-3-carbinol, and 3, 3′-diindolylmethane in mouse plasma, liver, and kidney tissues. J Chromatogr B. 2014;958:1–9.
Anderton MJ, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10(15):5233–41.
Anderton MJ, et al. Liquid chromatographic assay for the simultaneous determination of indole-3-carbinol and its acid condensation products in plasma. J Chromatogr B. 2003;787(2):281–91.
Staub RE, et al. Fate of indole-3-carbinol in cultured human breast tumor cells. Chem Res Toxicol. 2002;15(2):101–9.
Li Y, et al. Pharmacokinetic comparison of scutellarin and paeoniflorin in sham-operated and middle cerebral artery occlusion ischemia and reperfusion injury rats after intravenous administration of xin-shao formula. Molecules. 2016;21(9):1191.
Ramakrishna K, Srinivasan K, Sharma SS. Chronic treatment of 4-phenylbutyric acid ameliorates cognitive impairment after focal cerebral ischemia/reperfusion injury in rats. Indian J Physiol Pharmacol. 2021;64(3):188–94.
Brand M, et al. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 2013;169:1–8.
Longa EZ, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91.
Belayev L, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–22 (discussion 1623).
Shah FA, et al. Melatonin protects MCAO-induced neuronal loss via NR2A mediated prosurvival pathways. Front Pharmacol. 2019;10:297.
Zhang W, et al. Omega-3 polyunsaturated fatty acids mitigate blood–brain barrier disruption after hypoxic–ischemic brain injury. Neurobiol Dis. 2016;91:37–46.
Maleki SN, Aboutaleb N, Souri F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J Chem Neuroanat. 2018;87:54–9.
FDA, US. Guidance for industry: bioanalytical method validation (2018). Center for Drug Evaluation and Research (CDER), Silver Spring, MD and/or Center for Veterinary Medicine (CVM): Rockville, MD. 2001:1-27.
Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56(6):2795–805.
Grose KR, Bjeldanes LF. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992;5(2):188–93.
Deng Y, et al. Icariside II protects against cerebral ischemia–reperfusion injury in rats via nuclear factor-κB inhibition and peroxisome proliferator-activated receptor up-regulation. Neurochem Int. 2016;96:56–61.
Mukherjee S, Kumar G, Patnaik R. Withanolide a penetrates brain via intra-nasal administration and exerts neuroprotection in cerebral ischemia reperfusion injury in mice. Xenobiotica. 2020;50(8):957–66.
Jin G, et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39(9):2538–43.
Singh AA, et al. Biomedical application of Indole-3-carbinol: a mini-review. Phytochem Lett. 2021;41:49–54.
Stresser DM, et al. Substrate-dependent modulation of CYP3A4 catalytic activity: analysis of 27 test compounds with four fluorometric substrates. Drug Metab Dispos. 2000;28(12):1440–8.
Ahmad A, Sakr WA, Rahman K. Mechanisms and therapeutic implications of cell death induction by indole compounds. Cancers. 2011;3(3):2955–74.
Ramakrishna K, Singh N, Krishnamurthy S. Diindolylmethane ameliorates platelet aggregation and thrombosis: in silico, in vitro, and in vivo studies. Eur J Pharmacol. 2022;919:174812.
Acknowledgments
Kakarla Ramakrishna thanks IIT (BHU), Varanasi, and MHRD, India, for providing the teaching assistantship for carrying out the experimental work. We also thank Pankaj Paliwal and Akanksha Mishra, research scholars of Pharmaceutical Engineering and Technology, IIT (BHU), Varanasi, India, for their help in the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding.
Conflict of interest
The authors report no conflict of interest.
Ethics approval
The experimental protocol was approved by IMS, BHU, Varanasi (protocol no. Dean/2016/CAEC/31). The approved protocol strictly adhered to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and follows the ARRIVE guidelines.
Availability of data and material
All data generated or analyzed during this study are included in this manuscript and also available in a supplementary file.
Code availability
Not applicable.
Author contributions
KR designed and performed the experiment, analyzed the data, and wrote the manuscript. SKJ helped perform experiments and edited the manuscript. SK designed and analyzed the data and edited the manuscript.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramakrishna, K., Jain, S.K. & Krishnamurthy, S. Pharmacokinetic and Pharmacodynamic Properties of Indole-3-carbinol in Experimental Focal Ischemic Injury. Eur J Drug Metab Pharmacokinet 47, 593–605 (2022). https://doi.org/10.1007/s13318-022-00771-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00771-y