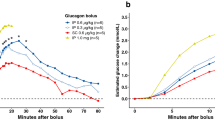
Abstract
Background and Objective
Artificial pancreases administering low-dose glucagon in addition to insulin have the scope to improve glucose control in patients with diabetes mellitus type 1. If such a device were to deliver both hormones intraperitoneally, it would mimic normal physiology, which may be beneficial. However, the pharmacokinetic properties of glucagon after intraperitoneal administration are not well known. Hence, the current study aims to evaluate the relationship between the amount of intraperitoneally delivered glucagon and pharmacokinetic variables in a pig model.
Methods
Pharmacokinetic data was retrieved from experiments on 19 anaesthetised pigs and analysed post hoc. The animals received a single intraperitoneal bolus of glucagon ranging from 0.30 to 4.46 µg/kg. Plasma glucagon was measured every 2–10 min for 50 min.
Results
Peak plasma concentration and area under the time–plasma concentration curve of glucagon correlated positively with the administered dose, and larger boluses provided a relatively greater increase. The mean (standard deviation) time to maximum glucagon concentration in plasma was 11 (5) min, and the mean elimination half-life of glucagon in plasma was 19 (7) min.
Conclusions
Maximum plasma concentration and area under the time–plasma concentration curve of glucagon increase nonlinearly in relation to the intraperitoneally administered glucagon dose. We hypothesise that the results are compatible with a satiable first-pass metabolism in the liver. Time to maximum glucagon concentration in plasma and the elimination half-life of glucagon in plasma seem independent of the drug dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
After intraperitoneal delivery, the time to peak plasma concentration and the elimination half-life of glucagon are dose-independent. Peak plasma concentration and area under the time–plasma concentration curve of glucagon increase with increasing intraperitoneal doses of glucagon. The relationship is nonlinear, which could indicate a satiable first-pass metabolism of glucagon in the liver. |
1 Introduction
Glucagon is secreted by the alpha cells of the pancreatic islets of Langerhans and glucagon-positive cells in the gastrointestinal tract [1]. Its main physiological effect is to stimulate hepatic glucose output in order to maintain euglycaemia [2, 3]. In individuals with diabetes mellitus type 1 (DM1), the alpha cells’ responsiveness to declining blood glucose concentrations is usually diminished. Consequently, they are susceptible to hypoglycaemia [4,5,6], which constitutes an important factor hampering optimal glycaemic control in insulin-treated patients with DM1 [5, 7].
Exogenous glucagon exerts the same pharmacodynamic effects as endogenous glucagon [8, 9], and rescue kits with high doses of glucagon, usually 1 mg, for intravenous, intramuscular or subcutaneous injection are commercially available and have been used for decades [10]. Lately, studies have demonstrated the potential benefit of delivering smaller doses of glucagon subcutaneously (SC) to counteract mild or impending hypoglycaemia in patients with DM1 [11,12,13]. Likewise, there is some evidence to support that the addition of glucagon leads to improved glucose control when utilised in SC bihormonal artificial pancreases, i.e. devices that both continuously monitor glucose levels and deliver insulin and glucagon subcutaneously [14,15,16,17].
Both insulin and glucagon are normally drained through the portal vein system after secretion from the pancreas. Thus, they reach the liver, a major site of action for both, before systemic circulation. An artificial pancreas delivering these hormones intraperitoneally (IP) could mimic normal physiology, as drugs administered IP are predominantly absorbed by the mesenteric vessels that empty in the portal vein [7, 18]. Insulin pumps with IP delivery have been tested on humans with some success [19, 20]. In contrast, the effects of IP-administered glucagon have only been studied in a few animal trials [21,22,23,24]. In a previous study on rats, our research group observed accelerated onset of action of glucagon after IP compared to subcutaneous (SC) administration [22]. In another study on pigs, we demonstrated superior glucose response after IP delivery compared to SC administration [21]. However, both of these studies focussed on the pharmacodynamics of IP glucagon. The pharmacokinetics of glucagon were not properly investigated.
A thorough knowledge of the pharmacokinetics of IP-delivered glucagon is essential for tailoring bolus sizes and administration intervals, and for designing control algorithms predicting the effects of glucagon on glucose levels in an IP bihormonal artificial pancreas. This knowledge is currently missing. Hence, the present study aims to determine the possible relationship between the size of a single IP-administered glucagon bolus and the following variables:
-
I.
The maximum glucagon concentration achieved in plasma (Cmax).
-
II.
The total drug exposure, as measured by area under the plasma glucagon time-concentration curve from 0 to the last measured time point (AUC0–last)
-
III.
The time it takes to reach maximum plasma concentration of glucagon (Tmax).
-
IV.
The elimination half-life of glucagon in plasma (T½) after peak plasma concentration is reached.
2 Methods
2.1 Data Collection
Data for this study were retrieved from four series of experiments conducted by our research group (Artificial Pancreas Trondheim, APT) between 12 April 2018 and 16 January 2020 [21]. In some experiments, the doses were fixed at 12, 50, 75 or 150 µg rather than based on weight. Thus, the total amount of glucagon delivered per kg bodyweight varied considerably. Pharmacokinetic parameters were not included in the original protocols, and, as such, were analysed post hoc. Some of the included pigs had received small doses of insulin prior to their glucagon bolus. Insulin, in contrast to glucagon, lowers blood glucose, and, as such, pharmacodynamic aspects could not be reliably assessed [9]. We have not found evidence of any pharmacokinetic drug–drug interactions between insulin and glucagon.
2.2 Animals and Animal Handling
In total, 19 pigs (8 males, 11 females) were included. Mean (SD) weight was 42.7 (5.3) kg. All the pigs were acquired from the same local supplier at around 12 weeks of age and brought to the research facility approximately 1 week in advance of the experiment to be acclimatised to the surroundings. They were fed commercial compound feed twice a day and provided with water without restriction. Food was removed in the evening approximately 10 h before the start of the experiments.
2.3 Experiment Protocols
2.3.1 Premedication and Anaesthesia
The pigs were premedicated before intubation with an intramuscular injection of either diazepam (Stesolid®; Actavis Group, Hafnarfjörður, Iceland) + azaperone (Stresnil®; Eli Lilly Regional Operations, Austria) + ketamine (Ketalar®; Pfizer, Norway), xylazine (Xysol®; CP-Pharma Handelsges, Germany) + ketamine or midazolam (Accord Healthcare, Harrow, UK) + ketamine. Anaesthesia was induced by an intravenous (IV) infusion of fentanyl (Actavis Group) + thiopental (VUAB Pharma, Roztoky, Czech Republic) and maintained by continuous IV infusion of midazolam + fentanyl and inhalation of isoflurane (Baxter, Oslo, Norway). Intubation was eased by an IV infusion of atropine (Takeda, Asker, Norway). The pigs received an IV infusion of the antibiotic cephalothin (Villerton Invest, Luxembourg) after anaesthesia was established. They were euthanised while still under anaesthesia at the end of the study day with an IV infusion of phenobarbital (NAF; Apotek, Lørenskog, Norway).
2.3.2 Suppression of Endogenous Glucagon Secretion
The effectiveness of somatostatin analogue treatment (SAT) has been evaluated in a previously published study by our research group [21]. The pigs were given SAT through either an IV infusion of 0.4 mg octreotide (Sandostatin®; Novartis Europharm, UK) every hour or a continuous IV infusion of 150 µg octreotide per hour to suppress their endogenous glucagon secretion. In addition, the first seven pigs received a subcutaneous injection of 0.3 mg pasireotide (Signifor®; Novartis Europharm) every third hour. The protocol was changed for later experiments due to a lack of support for an additional benefit of pasireotide.
The effectiveness of SAT in the present study was assessed by comparing the mean plasma glucagon concentration in two consecutive samples drawn before initiation of SAT with the mean of the last two quantifiable glucagon concentrations measured after SAT and before administration of exogenous glucagon. For one pig (pig 14), only one sample had been drawn before initiation of SAT, and, in this case, the single, known value was used. The overall mean (SD) plasma glucagon concentration before SAT was 6.3 (3.3) pmol/L, whereas the mean (SD) before glucagon administration was 4.3 (4.3).
2.3.3 Glucagon Delivery and Blood Sampling
Freshly constituted glucagon (Novo Nordisk, Denmark) was stored at room temperature and delivered via a pump to the peritoneal cavity. The administered IP dose varied between 0.30 and 4.46 µg/kg and was followed by arterial blood sampling every 2–10 min for 50 min. However, for one pig (pig 8), no sample was drawn at 50 min. The last sample used in the analyses for this pig was drawn at 46 min. The samples were stored in ice water for 10 min before centrifugation. Plasma was then transferred to Eppendorf tubes and stored at – 18 °C until the end of the experiment day. Afterwards, the tubes were kept at – 80 °C until analysis. Glucagon was analysed in singles with Glucagon ELISA kits (10-1281-01; Mercodia, Uppsala, Sweden). The inter-assay coefficient of variation was 9–27%.
2.4 Data Analysis
2.4.1 Pharmacokinetic Analysis
The reported concentrations are not baseline corrected, as the baseline concentration of glucagon was missing or not quantifiable in six pigs. Cmax and Tmax were obtained directly from the measured values. T½ and AUC0–last were estimated using the programme package Simbiology in MATLAB v.R2020B [25]. To calculate AUC0–last, the linear trapezoid method was used. For the six pigs where the baseline concentration was unobtainable, the average baseline value from the other 13 pigs was used at time zero in the AUC calculations (mean 4.3 pmol/L, SD 3.9). The terminal rate constant (λz), describing the decrease of the log-concentration of glucagon, was calculated by applying a best-fit linear regression to the terminal portion of the curve. The elimination half-life was calculated as ln2/λz. In three pigs (nos. 3, 4 and 6), no apparent elimination of glucagon after Cmax was observed, causing a negative value for T½. These pigs were therefore excluded from the half-life analysis.
2.4.2 Statistical Analysis
Statistical analysis was performed using GraphPad Prism v.9 [26]. Spearman’s rank-order correlation test was used to determine a possible correlation between dose size and pharmacokinetic outcomes. A 95% confidence interval (95% CI) not containing zero was considered significant. Means and standard deviations (SD) and medians and interquartile ranges (IQR) of Tmax and T½ were calculated. Possible outliers were identified through the ROUT method [27], using a q value of 0.01. Normality and log-normality were tested using the D’Agostino and Pearson test [28]. Correlation analyses were performed on non-transformed data. Because of the log-normal distribution, a logarithmic transformation was performed before linear regression analysis in order to make equations for Cmax and AUC0–last.
3 Results
Most of the pigs displayed a prominent rise and subsequent fall in glucagon concentrations after IP drug administration (Fig. 1), although some deviated from this pattern (Fig. 2).
Without correction, the mean (SD) Tmax was 13 (8) min, and the median value (IQR) of Tmax was 12 (8–15) min. After removal of two identified outliers [pig 5 (35 min) and pig 15 (32 min)], the mean (SD) was 11 (5) min, whereas the median (IQR) was 10 (8–15) min.
Simple linear regression after logarithmic transformation provided the following equation (Eq. 1) for Cmax (Fig. 3):
The 95% CIs of the intercept and slope were 2.54–3.47 and 0.06–0.46, respectively.
Simple linear regression after logarithmic transformation provided the following equation (Eq. 2) for AUC0–last (Fig. 4):
The 95% CIs of the intercept and slope were 5.94–6.86 and 0.08–0.47, respectively.
Without correction, the mean (SD) T½ was 23 (16) min, and the median value (IQR) of T½ was 19 (14–22) min. After removal of one identified outlier [pig 8 (77 min)], the mean (SD) was 19 (7) min, whereas the median (IQR) was 19 (13–22) min.
Tmax did not correlate with the glucagon dose, with a Spearman’s rank-order correlation coefficient (ρ) (95% CI) of 0.37 (− 0.14 to 0.73). Maximum plasma concentration was significantly correlated with dose size, with a ρ of 0.56 (0.13–0.81). AUC0–last was significantly correlated to dose size, with a ρ of 0.65 (0.26–0.86). The elimination half-life of plasma glucagon did not correlate with dose size, with a ρ of − 0.07 (− 0.57 to 0.47).
4 Discussion
The present analysis supports that there is a relationship between IP glucagon dosage and Cmax (Eq. 1) and AUC0–last (Eq. 2). Both equations are logarithmic, which suggests a linear curve with a breakpoint, where larger doses provide a relatively greater increase than smaller doses. This is compatible with a first-pass effect of glucagon in the liver that larger doses could saturate, as is observed with insulin [29, 30]. Because only a few or just one pig received the same dose, we cannot exclude the possibility that the findings are due to inter-individual variation. However, if a considerable presystemic hepatic metabolism of glucagon truly exists, it could support an IP versus a SC bihormonal artificial pancreas approach, as IP delivery may then lower the amount of glucagon entering the systemic circulation and hence possibly reduce the risk of adverse effects. This adds to our previous observation that, compared to SC glucagon delivery, half the dose given IP will achieve the same glucose-increasing effect [21]. More extensive, controlled studies with standardised doses are needed to investigate this further.
A majority of the pigs had a fairly consistent rise and fall in plasma glucagon concentration within 50 min after IP administration of the drug (Fig. 1). However, five pigs deviated from this pattern (Fig. 2). Pigs 3 and 4 (dosage 0.3 µg/kg) only achieved a minor increase in plasma glucagon concentrations compared to their baseline values, and clear elimination could not be observed after Cmax. The curves of pigs 6 (dosage 0.6 µg/kg) and 8 (dosage 1.04 µg/kg) also flattened after their peak, strongly affecting the observed half-life. Pig 15 (dosage 3.37 µg/kg) had a second, more prominent peak after the fall following the first peak. There are several possible explanations for this inconsistency, other than a genuine inter-individual variation in absorption and metabolism of IP-delivered glucagon, among which we consider the following most plausible:
-
I.
The IP tubes delivering glucagon could have been blocked.
-
II.
The suppression of endogenous glucagon could have been insufficient at some point during the experiments.
-
III.
IP fluid may have accumulated during anaesthesia, possibly causing dilution and altered absorption of the delivered drug [31, 32].
-
IV.
Loculaments of glucagon-containing fluid could have been formed within the intraperitoneal cavity making them less available for absorption. They may later have dissolved, explaining a second peak.
Tmax and T½ did not seem to differ in relation to glucagon dosage. However, because of the short observation time, the values for T½ should be interpreted with caution. Other human studies have conveyed comparable or slightly larger values for Tmax after SC administration of considerably larger doses [33,34,35,36,37,38,39,40,41]. Although the values are not directly transferable, this may indicate non-superiority of the IP route compared to the SC route in regard to absorption rate to the systemic circulation.
This study is limited by several factors, among which small sample size, unknown baseline values for a considerable number of pigs, large dose variation, single analysis of samples, inconsistency in anaesthetic protocol and relatively short observation times are the most prominent. Larger studies with a more robust design should be conducted to obtain more reliable results. Furthermore, extended trials where the animals may be administered multiple doses of glucagon are needed to increase the knowledge of possible intra-individual variation.
5 Conclusions
The present study indicates a relationship between the IP glucagon bolus size and the maximum plasma concentration and total drug exposure. In contrast, the time to maximum plasma concentration and the elimination half-life of glucagon seem to be independent of the dose. The results could be compatible with a satiable hepatic first-pass effect of IP-delivered glucagon. While further research is needed, if confirmed, this would support the endeavour to develop an IP bihormonal artificial pancreas, as it would reduce the systemic drug load and hence the expected adverse effects of treatment compared to a SC approach.
Change history
03 October 2021
The original version is updated due to request on integrate of funding note.
References
Knop FK. EJE PRIZE 2018: a gut feeling about glucagon. Eur J Endocrinol. 2018;178(6):R267–80.
Downes MA. Glucagon. Emerg Med. 2003;15(5–6):480–5.
Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39.
Haymond MW, Liu J, Bispham J, Hickey A, McAuliffe-Fogarty AH. Use of glucagon in patients with type 1 diabetes. Clin Diabetes. 2019;37(2):162–6.
Chung ST, Haymond MW. Minimizing morbidity of hypoglycemia in diabetes: a review of mini-dose glucagon. J Diabetes Sci Technol. 2015;9(1):44–51.
Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(4108):171–3.
Ranjan A. Glucagon treatment in type 1 diabetes—with focus on restoring plasma glucose during mild hypoglycemia. Dan Med J. 2018;65(2):B5449.
The AHFS Drug Information. Glucagon. American Society of Health-System Pharmacists (ASHP). http://www.medicinescomplete.com/. Accessed 20 May 2020.
Clinical Pharmacology 2020 database. Glucagon. Elsevier, Inc. https://www.clinicalkey.com/pharmacology/login. Accessed 20 May 2020.
Slama G, Bennis D, Zirinis P. Modes of glucagon administration. In: Lefebvre. Springer; 2012. p. 159–69.
Hartley M, Thomsett MJ, Cotterill AM. Mini-dose glucagon rescue for mild hypoglycaemia in children with type 1 diabetes: the Brisbane experience. J Paediatr Child Health. 2006;42(3):108–11.
Haymond MW, DuBose SN, Rickels MR, Wolpert H, Shah VN, Sherr JL, et al. Efficacy and safety of mini-dose glucagon for treatment of nonsevere hypoglycemia in adults with type 1 diabetes. J Clin Endocrinol Metab. 2017;102(8):2994–3001.
Haymond MW, Redondo MJ, McKay S, Cummins MJ, Newswanger B, Kinzell J, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care. 2016;39(3):465–8.
Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Technol. 2010;4(6):1288–304.
Taleb N, Haidar A, Messier V, Gingras V, Legault L, Rabasa-Lhoret R. Glucagon in artificial pancreas systems: potential benefits and safety profile of future chronic use. Diabetes Obes Metab. 2017;19(1):13–23.
Haidar A. Insulin-and-glucagon artificial pancreas versus insulin-alone artificial pancreas: a short review. Diabetes Spectr. 2019;32(3):215–21.
Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501–12.
Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther. 1971;178(3):562–4.
Spaan N, Teplova A, Stam G, Spaan J, Lucas C. Systematic review: continuous intraperitoneal insulin infusion with implantable insulin pumps for diabetes mellitus. Acta Diabetol. 2014;51(3):339–51.
Dirnena-Fusini I, Åm MK, Fougner AL, Carlsen SM, Christiansen SC. Physiological effects of intraperitoneal versus subcutaneous insulin infusion in patients with diabetes mellitus type 1: A systematic review and meta-analysis. PLoS One. 2021;16(4):e0249611.
Åm MK, Dirnena-Fusini I, Fougner AL, Carlsen SM, Christiansen SC. Intraperitoneal and subcutaneous glucagon delivery in anaesthetized pigs: effects on circulating glucagon and glucose levels. Sci Rep. 2020;10(1):13735.
Dirnena-Fusini I, Åm MK, Fougner AL, Carlsen SM, Christiansen SC. Intraperitoneal, subcutaneous and intravenous glucagon delivery and subsequent glucose response in rats: a randomized controlled crossover trial. BMJ Open Diabetes Res Care. 2018;6(1):e000560.
Loxham SJ, Teague J, Poucher SM, De Schoolmeester J, Turnbull AV, Carey F. Glucagon challenge in the rat: a robust method for the in vivo assessment of Glycogen phosphorlyase inhibitor efficacy. J Pharmacol Toxicol Methods. 2007;55(1):71–7.
Zlotnik A, Gruenbaum BF, Klin Y, Gruenbaum SE, Ohayon S, Sheiner E, et al. The effects of insulin, glucagon, glutamate, and glucose infusion on blood glutamate and plasma glucose levels in naive rats. J Neurosurg Anesthesiol. 2011;23(4):323–8.
MATLAB version R2020b (9.9.0.1467703): MathWorks, Inc. www.mathworks.com. Accessed 2 Jan 2021.
GraphPad Prism version 9.0.0 (86) for macOS: GraphPad Software LLC. www.graphpad.com. Accessed 1 Nov 2020.
Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006;7(1):123.
D'Agostino R, Stephens M. Tests for the Normal Distribution. In: Goodness-Of-Fit Techniques. New York: Marcel Dekker; 1986. pp. 367-413.
Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda). 2019;34(3):198–215.
Dirnena-Fusini I, Åm MK, Fougner AL, Carlsen SM, Christiansen SC. Intraperitoneal insulin administration in pigs: effect on circulating insulin and glucose levels. BMJ Open Diabetes Res Care. 2021;9(1):e001929.
Åm MK, Kolle K, Fougner AL, Dirnena-Fusini I, Bosch PC, Ellingsen R, et al. Effect of sensor location on continuous intraperitoneal glucose sensing in an animal model. PLoS ONE. 2018;13(10):e0205447.
Schnoor J, Schreck R, Baumert JH, Grosse-Siestrup C, Rossaint R, Unger JK. Influence of differences in body weight and volume management on experimental results in porcine models. Int J Artif Organs. 2004;27(11):924–34.
Blauw H, Wendl I, DeVries JH, Heise T, Jax T. Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes Obes Metab. 2016;18(1):34–9.
Castle JR, Youssef JE, Branigan D, Newswanger B, Strange P, Cummins M, et al. Comparative pharmacokinetic/pharmacodynamic study of liquid stable glucagon versus lyophilized glucagon in type 1 diabetes subjects. J Diabetes Sci Technol. 2016;10(5):1101–7.
El Youssef J, Castle JR, Bakhtiani PA, Haidar A, Branigan DL, Breen M, et al. Quantification of the glycemic response to microdoses of subcutaneous glucagon at varying insulin levels. Diabetes Care. 2014;37(11):3054–60.
El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra.
Graf CJ, Woodworth JR, Seger ME, Holcombe JH, Bowsher RR, Lynch R. Pharmacokinetic and glucodynamic comparisons of recombinant and animal-source glucagon after IV, IM, and SC injection in healthy volunteers. J Pharm Sci. 1999;88(10):991–5.
Hövelmann U, Bysted BV, Mouritzen U, Macchi F, Lamers D, Kronshage B, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41(3):531–7.
Hövelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab. 2019;21(3):601–10.
Ranjan A, Schmidt S, Madsbad S, Holst JJ, Nørgaard K. Effects of subcutaneous, low-dose glucagon on insulin-induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab. 2016;18(4):410–8.
Simmons KL, Williams G. Attempts to accelerate glucagon absorption: effects of adding a vasodilator and of injection using a ‘sprinkler’ needle. Eur J Clin Invest. 1992;22(6):434–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The trials were conducted at the Comparative Medicine Core Facility (CoMed) at the Norwegian University of Science and Technology (NTNU). The authors would like to convey their gratitude to Oddveig Lyng for her invaluable contribution in the execution of the trials.
Author contribution
All authors contributed to the conception and design of the study. Marte K. Åm was responsible for the conduction of animal experiments and data collection. Ingrid Anna Teigen analysed and interpreted the data and wrote the first draft of the manuscript. All authors reviewed and commented on previous versions of the manuscript. All authors have read and approved the final version of the manuscript.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital). The Double Intraperitoneal Artificial Pancreas Project is part of the Centre for Digital Life Norway and is supported by the Research Council of Norway (grant numbers 248872 and 294828). The project is further supported by the Central Norway Regional Health Authority (grant number 2014/23166) and the Norwegian Medical Association, Johan Selmer Kvanes Endowment. None of the funders had any part in the collection, analysis or interpretation of data.
Conflict of Interests
The authors declare no conflict of interests.
Ethics Approval
The Norwegian Food Safety Authority pre-approved all the trials from which the analysed data were gathered (FOTS number 12948). The experiments were conducted in accordance with the "Norwegian Regulation on the Use of Animals in Research" and the 2010/63 EU directive on the "Protection of Animals Used for Scientific Purposes".
Data Availability Statement
Data supporting the findings are available from the corresponding author upon request.
Code Availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Teigen, I., Åm, M.K., Carlsen, . et al. Pharmacokinetics of Intraperitoneally Delivered Glucagon in Pigs: A Hypothesis of First Pass Metabolism. Eur J Drug Metab Pharmacokinet 46, 505–511 (2021). https://doi.org/10.1007/s13318-021-00692-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00692-2