Abstract
Objective
To evaluate the efficacy of pre-exchange transfusion albumin priming in neonates with non-hemolytic hyperbilirubinemia.
Design
Single center, randomized controlled trial.
Setting
Level III Neonatal unit.
Participants
Fifty healthy term and late preterm neonates with non-hemolytic hyperbilirubinemia requiring exchange transfusion.
Interventions
5 mL/kg of either 20% human albumin (n=23) or 0.9% saline (n=27) infusion one hour prior to exchange transfusion.
Main outcome measure
Post-exchange transfusion phototherapy duration.
Results
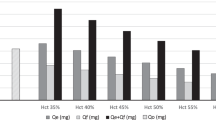
The post-exchange transfusion phototherapy duration was not different between albumin and saline groups [Median (IQR): 29 (24-48) h vs. 33 (24-43) h; P=0.76]. The total amount of bilirubin removed during exchange transfusion was also similar [Median (IQR): 34 (28-46) mg vs. 33 (27-38) mg; P=0.46]. Serial changes in total serum bilirubin following exchange transfusion and need for repeat exchange transfusion were comparable between the groups.
Conclusion
In healthy late preterm and term neonates with non-hemolytic hyperbilirubinemia, priming with 1 g/kg of 20% albumin prior to exchange transfusion is not superior to equivolume 0.9% saline in reducing post-exchange transfusion phototherapy duration or amount of bilirubin mass removed.
Similar content being viewed by others
References
Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. 2002;140:396–40.
Thayyil S, Milligan DW. Single versus double volume exchange transfusion in jaundiced newborn infants. Cochrane Database Syst Rev. 2006;4:CD004592.
Mitra S, Samanta M, Sarkar M, De AK, Chatterjee S. Preexchange 5% Albumin infusion in low birth weight neonates with intensive phototherapy failure -a randomized controlled trial. J Trop Pediatr. 2011;57: 217–21.
Shahian M, Moslehi MA. Effect of albumin administration prior to exchange transfusion in term neonates with hyperbilirubinemia -A randomized controlled trial. Indian Pediatr. 2010;47:241–244.
Odell GB, Cohen SN, Gordes EH. Administration of albumin in the management of hyperbilirubinemia by exchange transfusions. Pediatrics. 1962;30:613–21.
Kitchen WH, Krieger VI, Smith MA. Human albumin in exchange transfusion. A quantitative study of the influence of added human albumin on bilirubin removal. J Pediatr. 1960;57:876–83.
Tsao YC, Yu VY. Albumin in management of neonatal hyperbilirubinemia. Arch Dis Child. 1972;47:250–6.
Comley A, Wood B. Albumin administration in exchange transfusion for hyperbilirubinemia. Arch Dis Child. 1968;43:151–4.
Chan G, Schiff D. Variance in albumin loading in exchange transfusions. J Pediatr. 1976;88:609–13.
Ruys JH, Van Gelderen HH. Administration of albumin in exchange transfusion. J Pediatr. 1962;61:413–7.
Wood B, Comley A, Sherwell J. Effect of additional albumin administration during exchange transfusion on plasma albumin-binding capacity. Arch Dis Child. 1970;145:59–62.
Johnson L, Brown AK, Bhutani V. Bind. A clinical score for bilirubin induced neurologic dysfunction in newborns. Pediatr Suppl. 1999;104:746–7.
Mishra S, Agarwal R, Deorari AK, Paul VK. Jaundice in the newborns. Indian J Pediatr. 2008;75:157–63.
Variable Block Randomization Software, Available from: http://www.randomization.com. Accessed April 1, 201.
American Academy of Pediatrics. Provisional Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Saini SS, Kumar P, Balasubramanium K, Mehta S. Fluid supplementation in hyperbilirubinemia. Indian J Pediatr. 2011;78:1096–9.
Mehta S, Kumar P, Narang A. A randomized controlled trial of fluid supplementation in term neonates with severe hyperbilirubinemia. J Pediatr. 2005;147:781–5.
Lasky FD, Li ZM, Shaver DD, Savory J, Savory MG, Willey DG, et al. Evaluation of a bromocresol purple method for the determination of albumin adapted to the DuPont ACA discrete clinical analyzer. Clin Biochem. 1985;18:290–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dash, N., Kumar, P., Sundaram, V. et al. Pre-exchange albumin administration in neonates with hyperbilirubinemia: A randomized controlled trial . Indian Pediatr 52, 763–767 (2015). https://doi.org/10.1007/s13312-015-0713-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-015-0713-z