Abstract
Justification
Revised National Tuberculosis Control Program (RNTCP) has focused on adults with smear positivity — a tool not so well used in children with tuberculosis. There is a need to redefine standardization of diagnosis and management protocols for childhood tuberculosis.
Process
Indian Academy of Pediatrics constituted a Working Group to develop consensus statement on childhood tuberculosis (TB). Members of the Group were given individual responsibilities to review the existing literature on different aspects of the childhood TB. The group deliberated and developed a consensus which was circulated to all the members for review. Efforts were made to ensure that the recommendations are standardized.
Objectives
To produce recommendations and standard protocols for reasonably accurate diagnosis and rational treatment of tuberculosis in children.
Recommendations
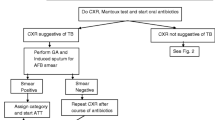
Fever and / or cough > 2 weeks with loss of weight and recent contact with infectious case should arouse suspicion of TB. Chest X-ray and trial with broad-spectrum antibiotic for 7–10 days is justified. In case of clinical and radiological non-response, Mantoux test and sputum or gastric aspirate for AFB is recommended. If AFB is positive, diagnosis is confirmed. If AFB is negative but chest X-ray is suggestive and Mantoux test is positive, it is a probable case and if these tests are negative, alternate diagnosis must be sought and referral made to an expert. Ideally it is recommended to use 1TU of PPD for Mantoux test but 2 or 5 TU may be acceptable (but less preferred). Cut-off point of 10 mms for natural infection may be used for test done with 1, 2 or 5 TU. There is no linear relation of reaction to tuberculin strength and so no more than 5 TU should be used. BCG test is not recommended. Diagnosis must not be made without an attempt to look for AFB in gastric aspirate or sputum, as it is possible to get AFB even in primary complex. Elisa and PCR tests for TB are not recommended. There is no place for trial of anti-tubercular therapy.
Lymphnode enlargement >2 cm with or without typical findings suggestive of TB and failure of antibiotic response demands FNAC for histopathology and bacteriology. Clinical suspicion of tubercular meningitis (TBM) should be confirmed by CSF examination and CT scan though none of these investigations are confirmatory and hence should not be considered in isolation. CSF tests for TB antibody and PCR are not recommended for routine use. Diagnosis of abdominal TB is made on circumstantial evidence and there are no standard guidelines.
For treatment, disease is divided into three categories. The Category I and III are recommended for different types of new cases i.e. those who have received treatment for not more than 4 weeks. Category III includes primary pulmonary complex, one site peripheral lymphadenitis and pleural effusion, while all other forms of TB are included in Category I, that corresponds to smear positive TB in adults. This is because AFB is often found in many Category I disease in children. Category II includes defaulters, relapses and failure cases irrespective of the site of disease.
Standard protocol is followed for each of these categories. Intermittent thrice weekly therapy with higher dose has been found to be equally effective as daily therapy and so is recommended in DOTS — Direct Observed Therapy Short term. Compliance of treatment must be ensured. Repeat chest X-ray is ideal at the end of therapy. Liver function tests are not routinely recommended. Recommendations are also made for special situations such as MDRTB, TB and HIV and neonate born to mother suffering from TB.
Similar content being viewed by others
References
IAP Working Group. Treatment of childhood tuberculosis: consensus statement of IAP working group. Indian Pediatr 1997; 34: 1093–1097.
IAP Working Group. Consensus statement of IAP Working Group: status report on diagnosis of childhood tuberculosis. Indian Pediatr 2004; 41: 146–155.
Management of Pediatric Tuberculosis under the Revised National Tuberculosis Control Program (RNTCP). A joint statement of the Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, and experts from Indian Academy of Pediatrics. Indian Pediatr 2004; 41: 901–905.
Chadha VK. Tuberculin test. Indian J Pediatr 2001; 68: 53–58.
Araujo Z, de Waard JH, de Larrea CF, Borges R, Convit J. The effect of Bacille Calmette-Guérin vaccine on tuberculin reactivity in indigenous children from communities with high prevalence of tuberculosis. Vaccine 2008;16:26: 5575–5581.
Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 2002; 57: 804–809.
Singla M, Sahai V, Sodhi S, Gupta RP. BCG skin reaction in mantoux negative healthy children. BMC Infect Dis 2005; 5: 19–20.
Somu N, Swaminathan S, Paramasivan CN, Vijayasekaran D, Chandrabhooshanam A, Vijayan VK, et al.. Value of bronchoalveolar lavage and gastric lavage in the diagnosis of pulmonary tuberculosis in children. Tuber Lung Dis 1995; 76: 295–299.
Singh M, Moosa NV, Kumar L, Sharma M. Role of gastric lavage and broncho-alveolar lavage in the bacteriological diagnosis of childhood pulmonary tuberculosis. Indian Pediatr 2000; 37: 947–951.
Lobato MN, Loeffler AM, Furst K, Cole B, Hopewell PC. Detection of Mycobacterium tuberculosis in gastric aspirates collected from children: Hospitalization is not necessary. Pediatrics 1998; 102: e40.
Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005; 365: 130–34.
Ichhpujani RL, Agarwal SP, Chauhan LS. Diagnostic needs and status of new diagnostic tools for tuberculosis. In: Agarwal SP, Chauhan LS. Tuberculosis control in India. Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. New Delhi: 2005. p. 165–178.
Dheda K, Udwadia ZF, Hugget JF. Utility of antigen-specific interferon gamma assay for the management of tuberculosis. Curr Opin Pulm Med 2005; 11: 195–202.
Bianchi L, Galli L, Moriondo M, Veneruso G, Becciolini L, Azzari C, et al.. Interferon-gamma release assay improves the diagnosis of tuberculosis in children. Pediatr Infect Dis J 2009; 28: 510–514.
Kampmann B, Whittaker E, Williams A, Walters S, Gordon A, Martinez-Alier N, et al.. Interferongamma release assays do not identify more children with active TB than TST. Eur Respir J 2009; 33: 1374–1382.
Kabra SK, Lodha R, Seth V. Some current concepts on childhood tuberculosis. Indian J Med Res 2004; 120: 387–397.
Verma K, Kapila K. Aspiration cytology for diagnosis of tuberculosis-perspectives in India. Indian J Pediatr 2002; 69 Suppl 1: S39–43.
Sharma M, Agarwal S, Wadhwa N, Mishra K, Gadre DJ. Spectrum of cytomorphology of tuberculous lymphadenitis and changes during antitubercular treatment. Cytopathology 2007; 18: 180–183.
El Jahiri Y, Chellak S, Garcia C, Ceppa F, Burnat P. The usefulness of adenosine deaminase determination in biological fluids for tuberculosis diagnosis. Ann Biol Clin 2006: 64; 117–124.
Kaur A, Basha A, Ranjan M, Oommen A. Poor diagnostic value of adenosine deaminase in pleural, peritoneal and cerebrospinal fluids in tuberculosis. Indian J Med Res 1992; 95: 270–277.
Gambhir IS, Mehta M, Singh DS, Khanna HD. Evaluation of CSF-adenosine deaminase activity in tubercular meningitis. J Assoc Physicians India 1999; 47: 192–194.
Jain R, Sawhney S, Bhargava DK, Berry M. Diagnosis of abdominal tuberculosis: sonographic findings in patients with early disease. AJR 1995; 165: 1391–1395.
Mwandumba HC, Squire SB. Fully intermittent dosing with drugs for treating tuberculosis in adults. Cochrane Database Syst Rev. 2001;(4):CD000970.
Kabra SK, Lodha R. Seth V. Category based treatment of tuberculosis in children. Indian Pediatr 2004; 41: 927–937.
Blomberg B, Spinaci S, Fourie B, Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull WHO 2001; 79: 61–68.
Kumar P. Journey of tuberculosis control movement in India: national tuberculosis control program to revised national tuberculosis control program. Indian J Tuberc 2005; S2: 63–71.
Kelkar-Khambate A, Klelmann K, Pawar S, Porter J, Inamdar V, Datye A, et al.. India’s Revised National Tuberculosis Control Program: looking beyond detection and cure. Int J Tuberc Lung Dis 2008; 12: 87–92.
Chugh S. Paediatric tuberculosis and DOTS strategy under RNTCP. J Indian Med Assoc 2008; 106: 799–802.
Enarson DA. The International Union Against Tuberculosis and Lung Disease. Model National Tuberculosis Programmes. Tuber Lung Dis 1995; 76: 95–99.
Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974; 99: 131–138.
Munoz FM, Starke JR. Tuberculosis in children. In: Reichman LB, Hershfield ES, editors. Tuberculosis: A Comprehensive International Approach: NewYork. Marcell Dekker Inc: 2000. p. 553–595.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amdekar, Y.K. Consensus statement on childhood tuberculosis. Indian Pediatr 47, 41–55 (2010). https://doi.org/10.1007/s13312-010-0008-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-010-0008-3