Abstract
In the face of a global epidemic of drug addiction, neglecting to develop new effective therapies will perpetuate the staggering human and economic costs of substance use. This review aims to summarize and evaluate the preclinical and clinical studies of deep brain stimulation (DBS) as a novel therapy for refractory addiction, in hopes to engage and inform future research in this promising novel treatment avenue. An electronic database search (MEDLINE, EMBASE, Cochrane library) was performed using keywords and predefined inclusion criteria between 1974 and 6/18/2021 (registered on Open Science Registry). Selected articles were reviewed in full text and key details were summarized and analyzed to understand DBS’ therapeutic potential and possible mechanisms of action. The search yielded 25 animal and 22 human studies. Animal studies showed that DBS of targets such as nucleus accumbens (NAc), insula, and subthalamic nucleus reduces drug use and seeking. All human studies were case series/reports (level 4/5 evidence), mostly targeting the NAc with generally positive outcomes. From the limited evidence in the literature, DBS, particularly of the NAc, appears to be a reasonable last resort option for refractory addictive disorders. We propose that future research in objective electrophysiological (e.g., local field potentials) and neurochemical (e.g., extracellular dopamine levels) biomarkers would assist monitoring the progress of treatment and developing a closed-loop DBS system. Preclinical literature also highlighted the prefrontal cortex as a promising DBS target, which should be explored in human research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Addictive substances pose significant socioeconomic impact on society and the healthcare system, costing hundreds of billion dollars annually in the USA alone [1, 2]. Psychological and adjunctive pharmacological treatments are the current mainstay for addiction therapeutics. However, the risk of relapse remains high, with some citing relapse rates as high as 75 to 98% within 1 year of treatment [3]. Investigations into new treatment modalities are urgently warranted. To develop novel therapies for addiction, researchers are focusing on the behavioral and neuroscience aspects in the biopsychosocial model (Fig. 1). Deep brain stimulation (DBS) in particular is a neuroscience-based potential treatment for medically refractory addictive disorders, given its diminishing surgical risks. “Medically refractory” cases have no formal definition, although these are generally regarded when conventional treatments repeatedly fail [4].
Biopsychosocial model depicting the different dimensions in consideration of addiction treatment. The stages of the addiction cycle of behavior are shown (inset) [140]. Interactions between components in different domains lead to addictive behaviors. Created with Biorender.com
Recent advances in neuromodulation techniques and devices led the indication of DBS to include movement disorders, such as Parkinson’s disease, to neuropsychiatric conditions, such as depression, Tourette’s syndrome, and obsessive–compulsive disorder (OCD) [5,6,7,8]. The use of DBS to reduce substance use has also been explored in both preclinical and clinical settings [9,10,11,12], offering a beacon of hope to patients suffering from medically refractory addictive disorders. This review aims to summarize and evaluate the potential therapeutic efficacy, mechanisms of action, and biomarkers involved in the use of DBS in the treatment of addictive disorders. We cautiously report that DBS is promising to treat certain refractory addictive disorders. Recommendations for the future directions are also provided.
Addiction Neurobiology: a Brief Overview
Addiction is a highly complex process involving multiple neurochemicals and brain structures. A highly simplified model highlights the central role of dopamine in mediating drug reward learning and drug seeking (compulsive) behaviors (Fig. 2). In essence, addictive drugs, such as the psychostimulants and opioids, enhance synaptic concentrations of dopamine in forebrain subcortical structures, such as the nucleus accumbens (NAc) [13]. These drugs of abuse significantly enhance dopamine concentrations over and above the ability of natural rewards, hijacking the system and encoding a more biologically salient reward association with surrounding environmental cues [13]. Such Pavlovian drug-environmental conditioning can trigger the desire to seek more of the drug. For example, the drug associated cues can elicit a surge of dopamine, which may manifest in drug craving, seeking and ultimately use [14].
A simplified illustration of the neurotransmitters and neuroanatomical structures involved in the pathophysiology of addiction [13, 19, 20]. Other relevant areas are omitted for clarity. The effect of dopamine receptors D1-R and D2-R is shown in inset. D1-R mediates the direct pathway (positive reinforcement) and D2-R mediates the indirect pathway (negative reinforcement). Italic descriptions denote changes secondary to chronic drug use. 5-HT, serotonin; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; D1/2R, dopamine 1/2 receptors; DA, dopamine; GABA, gamma aminobutyric acid; NAc, nucleus accumbens; NMDAR, N-methyl-d-aspartate receptor; VTA, ventral tegmental area. Created with Biorender.com
Serotonin (5-HT) is another important neurotransmitter mediating neuroplasticity, hedonic tone, motivational, and reinforcement processes, as well as cognitive functions [15, 16]. 5-HT2A and 5-HT2C receptors in the prefrontal cortex (PFC), NAc, and ventral tegmental area in particular appear to be critical. 5-HT2A receptor increases, and 5-HT2C receptor decreases, striatal dopamine release [15]. The serotonergic neuroadaptations may be accountable for emotional components such as anhedonia and depression during drug withdrawal [16]. Recent evidence in mice also suggest that serotonin signaling via 5-HT1B receptor may attenuate transition from casual to compulsive cocaine use [17].
Chronic drug use ultimately alters the homeostatic neuroplasticity in the brain which not only results in a sensitized dopaminergic system [18], but also leads to changes in the glutamatergic system [13, 19, 20]. For example, calcium-permeable ionotropic glutamatergic AMPA receptors become upregulated relative to glutamatergic NMDA receptors [13, 19, 20]. The changes in AMPA and NMDA receptors have become a hallmark process of neuroplasticity that underpins behavioral changes following chronic drug use [21]. Another neurobiological adaptation that occurs with chronic drug use is the process of “dorsalization” [22]. Here, repeated exposure to drugs of abuse leads to recruitment of the circuit that projects from the orbitofrontal cortex (OFC) to the dorsal striatum (caudate and putamen), where synaptic potentiation occurs at both dopamine receptors 1 and 2 (D1-R and D2-R) on GABAergic medium spiny neurons (MSNs).
Activation of D1-Rs receptors causes excitation in the postsynaptic cell via cyclic adenosine monophosphate production (cAMP) production [23] that ultimately leads to promotion of long-term potentiation [24]. D1-R- and NMDA receptor–dependent activation of extracellular signal-regulated kinase (ERK) in brain areas such as striatum is an important pathway for drug-induced locomotor response and sensitization [25,26,27,28]. For example, systemic injection of D1-R- or NMDA antagonist prevents ERK phosphorylation triggered by d-amphetamine in the mouse striatum [29]. In addition, cocaine-induced ERK phosphorylation and locomotor sensitization is prevented with mitogen-activated protein kinases/ERK kinase inhibitor or in mutant mice with alanine-replaced Thr-34 residue of dopamine- and cAMP-regulated phosphoprotein (DARPP-32) [29]. D1-R MSN structural plasticity depends on NMDA and DARPP-32 signaling [24]. Psychostimulants appear to affect ERK signaling via DARPP-32-based D1-R- and NMDA intracellular pathways [29].
Importantly, D1-R activation inhibits long-term depression, as revealed by D1-R antagonist unmasking long-term depression [30]. In contrast, D2-Rs can promote long-term depression and prevent the induction of long-term potentiation [30]. However, D2-Rs are particularly sensitive to dips in the tonic dopamine level, during which they activate protein kinase A (PKA) to induce long-term potentiation [30,31,32]. Taken together, D1-R and D2-R are critical for bidirectional plasticity of striatal MSNs when dopamine levels are high vs low to guide adaptive and maladaptive behaviors involved in reward learning [31, 32]. Therefore, D1-R and D2-R signaling dynamically modulates cortico-striatal plasticity depending on the level of synaptic dopamine to facilitate transition into persistent drug seeking and use.
While DBS appears to target these processes involved in addiction to reduce drug seeking and taking [10, 33,34,35,36,37], the potential therapeutic efficacy and mechanisms of action of DBS in the treatment of addictive disorders are poorly understood. We address this gap in the present review.
Methods
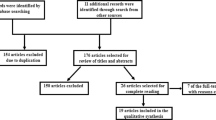
A search was performed using keywords, such as “deep brain stimulation” and “addiction” in MEDLINE, EMBASE, and Cochrane Library database between 1974 and 6/18/2021. Details of search criteria and inclusion and exclusion criteria are given in Supplementary Information Appendix A. Search results are presented in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart (Fig. 3). This review was registered at the Open Science Framework [38]. The process yielded 47 papers (25 preclinical studies and 22 human studies), which were further reviewed in full text. The results of this literature search are summarized in the sections below.
Results and Discussion
Preclinical Models and Clinical Correlation
Animal DBS studies in the selected literature (Table 1) predominantly used conditioned place preference (CPP) or self-administration paradigms (Fig. 4) to model addiction. Self-administration models have high construct, content, and face validity relative to human addictive behaviors. Experimenter-administration models such as CPP can measure drug-induced behavioral sensitization, such as increased locomotion, and are easier to perform. Both models also allow modeling of withdrawal symptoms during abstinence and/or extinction phase, followed by relapse behaviors, such as cue or drug-induced reinstatement. Most common DBS brain targets in the selected animal studies (Table 1) were the NAc [33, 39,40,41,42,43,44,45], PFC [44, 46, 47], and subthalamic nucleus (STN) [10, 37]. The following sections will focus on these three most studied targets.
Illustration of two most widely used experimental paradigms for the study of drug addiction in animals. On the left, conditioned place preference (CPP) is shown, where “non-contingent” drug-administration is associated with one side of the chamber during drug-side associated conditioning. If the drug is experienced as rewarding, the animal will spend more time in the drug-administered side relative to the other side, even in the absence of further drug administration. On the right, “contingent” drug self-administration paradigm allows the animal to regulate its own drug intake (e.g., via nose-poke or lever-pressing). A discrete light and/or tone cue may be provided in association with the drug intake to serve as a drug-associated cue. In extinction, the animal is placed back into the environment where CPP or self-administration is acquired but without drug availability. Extinction sessions lead to reduced place preference or drug seeking. During reinstatement, the drug, the drug-associated cue, and/or stress can be given, which can lead to “relapse” measured by preference or drug seeking. Created with Biorender.com
NAc Electrical Stimulation
The NAc is divided into core and shell subregions with different histopathological compositions, projections, and functions [48]. Studies suggest that the shell is responsible for reinforcing properties of novelty, rewards (both drug- and non-drug-related), and drug relapse, whereas the core mediates spatial learning, conditioned responses, responses to motivational stimuli, and impulsive choices [14, 49]. These features of motivated behaviors suggest NAc shell underlying extinction and reinstatement behavior [50], and NAc core underlying the initial acquisition of drug taking and cue-elicited drug seeking [48]. However, there is synergy between the two subnuclei, and both play a role in drug addiction.
Stimulation of either the core or shell subregions of the NAc using a range of low and high frequencies leads to reduced drug taking, seeking, or CPP in rats conditioned with alcohol [34, 51, 52], nicotine [53], opioids [9, 37, 39, 41, 46, 54,55,56,57], cocaine [10, 33, 35, 40, 42, 43, 47, 58, 59], or methamphetamine [45] (Table 1). Behavioral improvement was related to the timing of when electrical stimulation was given—that is, stimulation given before a self-administration session or CPP acquisition would reduce drug intake or prevent CPP, respectively [39, 45]. When given during abstinence or reinstatement, stimulation would diminish drug or cue-induced drug seeking after extinction and/or forced abstinence phase [40,41,42,43, 45]. Such efficacy of stimulation during abstinence to prevent relapse is particularly promising in the clinical context.
While most animal and human studies (Table 2) show success in modulating addictive behavior using NAc stimulation, there are two main issues. First, there is conflicting evidence in animal studies regarding the efficacy of NAc core vs shell stimulation. Both high- and low-frequency stimulation (HFS, LFS) of NAc (electrodes placed across core and shell) can reduce cocaine-primed drug seeking following 24 days of abstinence [43]. Other studies using core as the DBS target showed reduced morphine-induced CPP [39], ethanol self-administration [51], and heroin reinstatement [41]. The shell was also shown to be an effective target for morphine-induced CPP [55], cocaine reinstatement [40], ethanol self-administration [44, 51, 52], and methamphetamine reinstatement [45]. In contrast, Vassoler et al. demonstrated that stimulation of the NAc shell but not the core attenuated cocaine-induced reinstatement [42].
The contradicting evidence above may be explained by the large activation volume of stimulation pulses, such that pulses centered on the core may also affect the shell, and vice versa. From human studies, the volume of activation was estimated to be between 30 and 120 mm3 [60] and the human NAc volume is around 700 mm3 [61]. However, for adult Sprague–Dawley rats, the total volume of NAc is approximately 5 mm3 with the NAc core making up half of that [62]. Therefore, neighboring structures could be co-stimulated in rats. Notably, the core and shell divide is functionally present in humans while they are less distinct in rodents [63, 64].
The second issue concerns laterality. While most studies used bilateral stimulation, some studies only used unilateral NAc stimulation [34, 39, 41, 43]. One study used left, right, and bilateral NAc core stimulation, demonstrating only the latter two were effective in reducing heroin seeking behavior [41]. It is unclear why such asymmetry exists. Dopamine concentration in the NAc is reported to be higher on the side ipsilateral to the paw-dominance of a mouse [65]; however, morphometric NAc human studies based on laterality have been controversial [49].
Almost all of these published studies in humans show some improvement from DBS in the NAc in addiction-related behaviors (Table 2) as well as cognitive improvement, which raises the possibility of potential publication bias. With this precaution in mind, NAc DBS is likely the lowest hanging fruit for refractory addictive disorders. A recent systematic review supports NAc DBS to treat substance use disorders, demonstrating that NAc DBS in patients led to a relapse rate of ~ 39% with a reasonable safety profile, which is an improvement compared to the ~ 60% relapse rate reported in that population (selection bias factors notwithstanding) [66]. In addition, individual case reports revealed that those with comorbid psychiatric disorders, such as depression and OCD, showed improvement in these other symptoms with NAc DBS with or without anterior limb of internal capsule (ALIC) intervention (DBS or capsulotomy) [12, 67, 68]. DBS may provide additional benefit for patients with multiple psychiatric comorbidities.
Cortical Stimulation
The PFC is believed to provide top-down control of NAc in addiction (Fig. 2). DBS of the PFC (subregion not specified) significantly reduced cocaine seeking and motivation to take cocaine measured by progressive ratio test [35]. Stimulation of the infralimbic cortex of the PFC following a cocaine prime also reduced cocaine reinstatement [47]. Notably, mPFC stimulation in rats showed increased phasic dopamine release in the NAc, which might explain the stimulation effect [69]. However, stimulation of the infralimbic cortex starting 1 h or 4 days before alcohol prime did not affect alcohol-induced relapse following abstinence in rats [44]. The contrasting findings may be due to the different mechanisms of actions of the drugs being given (alcohol is a depressant whereas cocaine is a stimulant). Alternatively, infralimbic cortex stimulation may be more effective in reducing relapse-like behaviors following extinction rather than forced abstinence, consistent with the well-known role of the infralimbic cortex in extinction [70]. Infralimbic cortical DBS success is concordant with reduced craving by transcranial direct current stimulation (tDCS) of dorsolateral PFC in humans [71]. However, DBS of the prelimbic cortex following cocaine priming had no effect on cocaine reinstatement in rats [47].
In the OFC, DBS prevented rats from learning morphine-induced CPP, while DBS during extinction facilitated extinction acquisition and prevented morphine-induced reinstatement [9]. Chronic DBS of the anterior insular cortex either during 11 days of extinction or 14 days of abstinence reduced CPP, but CPP relapsed 10 days after chronic DBS [57]. This finding is consistent with how repetitive transcranial magnetic stimulation (rTMS) reduced heavy drinking only during the intervention but not after in alcohol-dependents [72]. The insula’s superficial location and delicate anatomy may be better suited for non-invasive interventions such as rTMS rather than DBS.
Anterior cingulate cortex (ACC) dysfunction can disrupt ongoing processing and detection of erroneous outcomes relevant for reward learning [73, 74]. ACC DBS following cocaine prime did not alter reinstatement following self-administration in rats [47]. However, when people with alcohol dependence received bilateral DBS (6- or 10-Hz stimulation), craving was significantly reduced [75]. The reduced craving was associated with a reduction in beta-1 band (13–18 Hz) current density on electroencephalogram (EEG) post-stimulation in the rostrodorsal ACC [75]. Alcohol craving also correlated with beta activity in the dorsal ACC in a case report, although relapse was associated with increased gamma band activity [76]. Another case report showed that a patient with concomitant OCD and alcohol dependence was successfully treated with ACC rTMS [77]. Interestingly, NAc DBS reduced craving correlated with improvement in error-related negativity in LFP in the ACC in humans [78], suggesting the effect of NAc DBS may alleviate ACC dysfunction. In addition to DBS, rTMS of the dorsal ACC demonstrated improvement in alcohol craving and concomitantly reduced NAc activation on fMRI [76]. Taken together, DBS in ACC may be particularly relevant for alcohol use.
Most of these cortical regions project to the NAc, raising the possibility that their DBS effects are mediated via these connections [49, 79]. Animal studies show not only strong projections from the cortex to NAc, but also a high degree of interplay between the two regions [80]. For example, c-Fos reactivity is observed in infralimbic cortex and NAc during DBS [42, 56] Pharmacological silencing of cortical interneurons can reinstate drug seeking [42, 81], which is consistent with silencing effects observed in the NAc [81]. Nevertheless, NAc is unlikely to be the sole driver of effects of DBS in the PFC considering that DBS in the prelimbic cortex does not affect drug seeking in rats, even though prelimbic cortex has strong connections to NAc [47].
STN Stimulation
Parkinson’s patients who take dopaminergic medications for their movement disorder may chronically feel compulsion to over-consume such agents, even at the expense of dyskinesia and other side effects. Studies suggest that STN DBS in humans may improve movement symptoms as well as reduce compulsive use of medications [82, 83].
STN DBS or lesions in rats have been shown to reduce motivation for cocaine taking, while increasing motivation to consume more common rewards such as food [10, 37, 59, 84]. The lesion study in particular suggests that STN DBS may work via local inactivation [59, 84]. Further evidence shows STN HFS in rats inhibiting substantia nigra, entopeduncular nucleus, and NAc shell measured with brain mapping analyses of immediate-early gene expression but not PFC, and silencing STN neurons measured using recording ex vivo [37].
Although HFS of STN in rats reduced both cocaine taking and heroin seeking [37, 59], a conflicting study demonstrated only LFS (30 Hz) but not HFS modulates cocaine taking behavior in the presence of foot-shock punishment [10]. Abnormal alpha/theta and low-beta oscillatory activity during escalation of the cocaine intake phase also predicted the subsequent emergence of compulsive-like seeking behavior, where the animals were shock-resistant [10]. The discrepancy here may be explained by the absence/presence of punishment during drug taking, but further studies are warranted to understand STN LFS vs HFS outcomes.
Other Brain Regions
Electrical stimulation targeting other brain regions in animals—amygdala [47, 85], hippocampus [47], periaqueductal gray matter [54], lateral hypothalamus [35], dorsal striatum [40, 44], and lateral habenula [58]—has also been described in the literature (Table 1). However, due to the limited number of studies per brain region, meaningful conclusions are difficult to draw. Hence, these regions are beyond the scope of this review.
High- Versus Low-Frequency Stimulations
Whereas most animal studies reported HFS (> 130 Hz) effects on behavior, some used a lower frequency (< 20 Hz) and observed beneficial effects targeting the NAc [43], lateral habernula [58], ventral striatum [56], and periaqueductal gray [54] even at frequencies as low as 8 Hz (STN) [10] (Table 1). In contrast, LFS targeting the NAc shell [33], PFC [35], or OFC [9] were ineffective. Interestingly, DBS at 12 Hz alone did not affect cocaine-induced locomotor sensitization, but combined with dopamine D1-R antagonist, reversed cocaine-induced hyperlocomotion and plasticity in the NAc shell [33]. Such findings suggest that LFS may be effective only when basal dopamine signaling is low.
The effect of HFS in NAc shell may be short-lived, lasting less than 4 h in a cocaine experimenter-administered mouse model [33] or 1 day in an alcohol self-administration rat model [34]. However, reduction in cue-induced seeking behavior was maintained more than 4 weeks post-HFS (NAc shell) compared to the sham group in rats that self-administered methamphetamine [45]. Yet, LFS and HFS (mixture of NAc core and shell) effects lasted at least 2 weeks but less than 30 days in a cocaine self-administration rat model [43]. The different DBS parameters, targets, drugs, rodents, and tests across these studies (Table 1) pose a challenge to evaluate the best DBS protocol to reduce drug use.
Potential Mechanism(s) of Action
Neuronal Inhibition
Despite the promising results of DBS in addiction, the mechanism of action is poorly understood and likely highly complicated. Several hypotheses on the mechanism of DBS in addiction have been suggested, such as direct inhibition/excitation of neural activity, information lesioning, and synaptic filtering where DBS blocks transmission of pathological low-frequency oscillations [86]. In particular, the inhibition hypothesis is supported by animal studies where both injection of GABA agonists (i.e., temporary inhibition) and HFS in the NAc was found to reduce alcohol use in rats [34]. Similar effects were also seen between STN lesion paradigms and DBS with cocaine conditioning [59, 84]. It is likely the neurons and their respective synaptic activities respond in a combination of different ways depending on the stimulation frequency and the drug being tested.
Depotentiation of Excitatory Inputs onto D1-R Via Modulation of Glutamate Receptors
Evidence also suggests the interaction between dopamine and glutamate receptors is involved in the mechanism of DBS action. For example, NAc shell LFS (12 Hz), only when used in combination with a D1-R antagonist, could reverse the motor sensitization and cocaine-evoked plasticity changes in D1-Rs on GABAergic MSNs, in the form of increases AMPA/NMDA glutamate receptor ratio [33]. By using a metabotropic glutamate receptor (mGluR1) antagonist to block the therapeutic effect, the authors proposed that the mechanism of action of DBS is mGluR-dependent, the activation of which depotentiates excitatory synaptic inputs onto D1-Rs on GABAergic MSNs [33]. Such depotentiation was associated with a normalization in drug-adaptive behavior. The findings are also consistent with the upregulation of GluR1 seen in the NAc in other stimulation studies [35, 36].
In contrast, both LFS (20 Hz) and HFS (160 Hz) stimulation of the NAc core attenuated cocaine seeking behavior in rats without the need of additional medications [43], similar to the outcome of PFC stimulation [35]. The contrasting findings between NAc core and PFC stimulations may be explained by the different levels of basal dopamine in these brain regions. Examining how DBS affects mGluR1 expression in different regions to affect D1-R signaling would be informative in future work.
Antidromic Activation of Cortex
Post-mortem brain c-Fos immunohistochemistry following in vivo NAc shell HFS showed both local activation and activation of the infralimbic cortex, suggesting that DBS of the NAc shell led to antidromic stimulation of the cortex via cortico-accumbal afferents [42]. Consistent with infralimbic cortex-NAc circuitry in DBS, electrophysiological studies showed NAc HFS but not LFS enhances both NAc-evoked LFP responses and spontaneous LFP slow oscillatory activity in OFC in an antidromic fashion [87]. These results further demonstrate the complex interplay between NAc and the cortex.
Dopamine Replacement
One study showed that HFS of the NAc shell led to a paradoxical augmentation in relapse behavior in a rat model of alcohol addiction [44], a contrary finding to other studies (Table 1). The authors also demonstrated an increase in extracellular levels of dopamine as measured by microdialysis. DBS-evoked increase in dopamine is reminiscent of the intracranial self-stimulation model, where the subjects self-administer electrical stimulation via implanted electrodes in the brain [88]. Reinforcing effects of electrical stimulation is presumably mediated by dopamine release in the reward circuitry [89]. In addition, medial PFC DBS evoked dopamine release in the NAc in a frequency-dependent manner in anesthetized rats measured by fast-scan cyclic voltammetry (FSCV) [69]. Taken together, DBS of PFC may operate partially via the replacement of dopamine, but whether this can reduce drug taking or seeking has yet been tested. Most of the DBS studies did not employ in vivo microdialysis techniques for direct quantification; hence, replacement of dopamine as a DBS mechanism is unconfirmed.
Limitations of Animal Models
Human subjects tend to require weeks and months of DBS to observe an improvement, whereas animal studies show an almost immediate effect. In animal studies, the conditioning occurs over days (rather than years). The short timeframe indicates that the neuroplasticity reversal may not be the dominating effect of DBS in animals and the dopamine replacement caused by stimulation may mediate the acute improvement in behavior. In humans, DBS likely operates via reversal of neuroadaptations that have been built up on a much longer time scale than animals subjected to conditioning. This interpretation is supported by case reports where subsequent explantation of the DBS device did not reverse the initial effect of abstinence [90, 91]. In addition, a further case report showed that NAc DBS reduced risk taking behavior and increased activation of brain regions implicated in behavioral control arising from lasting neuroadaptations, measured by positron-emission tomography [92].
Some human studies also include subjects with comorbid OCD, depression, and anxiety [12, 93, 94]. In fact, addiction may be part of a maladaptive coping mechanism for other underlying disease/s. DBS may alleviate addiction by reducing the severity of an underlying comorbid disease, which is challenging to model in animals. For example, the higher cognitive and emotional component as well as the impact of social cues are difficult to assess in animals.
Alternatives to DBS
Given the invasive nature of DBS, other neuromodulatory modalities are worth considering. Of note, noninvasive techniques such as rTMS and tDCS have been of particular interest [95, 96]. A detailed review of these techniques and outcomes is out of the scope of this article but in brief, rTMS operates by applying a stimulating coil at the scalp, which generates magnetic fields. The magnetic fields then pass through the skull and induce strong focal currents in the underlying brain tissues [97]. It is believed that long-term repetitive stimulation can lead to neuroplasticity, which can provide therapeutic effect to the addicted brain [97]. tDCS involves application of a direct or alternative electrical current to the scalp, such that current travels from a positive (cathode) to a negative (anode) electrode. Cortical neurons are suggested to be facilitated under the anode, whereas those under the cathode are suppressed [98]. Although rTMS and tDCS have been shown to be effective in preclinical studies and small clinical case series on reducing symptoms such as craving, more data are required to ascertain their long-term safety profiles, efficacy, and the ideal patient profile [98,99,100]. Although research shows that non-invasive neuromodulation can be successful in certain cases without the need for neurosurgery, the main limitations are the focality of these non-invasive treatments and the relatively low spatial resolution. These non-invasive interventions also require numerous frequent visits to facilities in a specialized, tertiary center. In contrast, DBS can be implanted as a permanent or chronic stimulation system, which can potentially be programmed remotely [101].
There was a recent success of semi-invasive MRI-guided focused ultrasound (MRgFUS) thalamotomy in the treatment of movement disorders and other psychiatric disorders (depression and OCD) [102, 103]. Such a technique would avoid the complications associated with the implantation of devices, which may necessitate explantation for reasons such as lack of efficacy or infection. Alongside DBS, MRgFUS offers a novel therapeutic avenue that should be explored to treat refractory addictive disorders.
Invasive techniques can offer a more focal region of therapy. Ablation techniques in drug addiction targeting the NAc with promising results have long received attention [104]. Indeed, a recent systematic review showed that NAc DBS has similar efficacy and safety profile as radiofrequency ablation of the NAc [66]. Compared to ablation techniques, however, DBS allows adjustment of stimulation parameters tailored to maximize therapeutic efficacy while minimizing side effects. Nonetheless, a recent systematic review showed that NAc DBS has similar efficacy and safety profile as radiofrequency ablation of the NAc [66].
Future Directions
Potential Biomarkers
The hunt for a reliable biomarker serves three critical purposes: to facilitate patient selection, to monitor the progress of the disease (and therapeutic effect of treatments), and to progress towards a holy grail of DBS—a closed-loop therapeutic system. Not only can this potentially increase the battery longevity (hence reducing the need for battery replacement), it also can increase the efficacy of stimulation by tailoring to the patients’ neural responses. Further, a “tolerance” effect can be observed with chronic open-loop DBS stimulation in movement disorder patients, with the stimulation effect wearing off after a period of time [105]. Such a tolerance is also likely in patients receiving DBS to treat psychiatric illnesses. Closed-loop system with intermittent, dynamic stimulation can potentially minimize tolerance. Such biomarkers need to be easy to measure and possess a relatively high spatiotemporal resolution of measurement that, in turn, correlates well with clinical/behavioral changes. In our opinion, the most promising candidates would be electrophysiological and neurochemical measurements, although they will need to be tailored towards individual addictive substances and patients.
In terms of electrophysiological measurements, LFP is the most widely studied potential biomarker among DBS studies. LFP’s wide use is mostly because of its measurement using the stimulating electrodes themselves. In urethane-anesthetized rats, NAc HFS suppressed pyramidal cell firing and enhanced slow LFP oscillations in the OFC via antidromic activation of cortico-striatal recurrent inhibition [87]. In addition, prolonged NAc HFS and LFS (delivered over 90 min) have been shown to affect both spontaneous and evoked LFPs in the medial PFC, lateral OFC, mediodorsal thalamus (MD), and NAc sites [106]. HFS also produced widespread increases in spontaneous beta and gamma power and enhanced coherent beta activity between MD and all other regions. In contrast, LFS elevated theta power in the MD and NAc. Acutely, HFS increased and LFS decreased induced relative gamma coherence. Stimulation-induced changes in gamma coherence shows that NAc DBS may achieve therapeutic effects by restoring the synchronicity of a neural circuitry that is disrupted in neuropsychiatric diseases. The differences in the effect on LFPs between HFS and LFS may also explain the differences in behavioral effects described above.
In a study assessing 7 people with heroin use disorder, pre-DBS electrophysiology revealed prominent theta and alpha band activity in both the NAc and ALIC [107]. Additionally, a beta band peak was detected in the power spectra of ALIC LFPs, which, the authors hypothesized, may represent the activity of striatal bridge cells. Furthermore, there was a negative correlation between the power of the theta band of ALIC LFPs and subjective craving, and a positive correlation between the power of the alpha band of NAc LFPs and depressive symptoms. LFPs of the NAc and ALIC both exhibited higher coherence values in the theta and alpha frequency bands. No comparison with post-DBS recordings was given in this study. Together with the animal and human DBS studies on NAc [108, 109], the most consistent biomarker frequency is yet unknown. Further studies are required for clarification. New technologies such as the Medtronic Percept Stimulator [110] may be able to provide more information by simultaneously recording from and stimulating brain targets in humans. In addition, trials investigating electrophysiological changes secondary to DBS in addiction (such as [111]) will provide more insights.
Unlike electrophysiological signals (i.e., action potentials) that naturally lead to neurotransmitter release into synapses, quantification of synaptic-related neurotransmitter extracellular concentrations directly measures the activation of relevant neural circuits (such as the ones depicted in Fig. 2). Most studies in addiction focus on the monoamines, particularly dopamine, glutamate, and 5-HT. One animal study reviewed here [44] employed in vivo microdialysis and found that local dopamine concentrations increased after NAc shell HFS. While in vivo microdialysis has long been the gold standard for sampling neurochemicals, the process necessitates an external analyzer and has low temporal resolution. In contrast, in vivo electrochemical techniques, such as FSCV, provide potential benefits such as higher temporal resolution, less tissue damage, and the ability to be chronically implanted [112,113,114,115,116]. However, compared to microdialysis, electrochemical techniques have other limitations. Often, they are restricted to measurement of a single electroactive amine at a time, such as dopamine or serotonin. Thus, future studies which aim to develop biomarkers for DBS efficacy will need to demonstrate suitability of neurochemical biomarkers for translation and may be limited by present detection technology. While microdialysis provides important measurements of the tonic (basal) extracellular levels of neurochemicals, voltammetry can provide measurement of phasic and tonic measures of neurochemicals [117,118,119,120,121,122].
Future Preclinical and Clinical Research Considerations
Other important considerations for future research include inclusion of both genders in both animal and human studies (currently most studies focus on males—see Tables 1 and 2), and the use of larger animals—preclinical studies are predominantly rodent-based, but also well suited to large animal models. In addition, we have focused on technologies that are currently clinically viable in this review, namely electrical DBS devices. However, the use of optogenetics, where a viral vector is able to transfect a specific neural population, is increasingly popular in the preclinical study of underlying pathophysiology of different neuropsychiatric disorders and relevant therapies. Optogenetics has provided new insights into DBS protocol optimization for Parkinson’s disease with translational potential [123, 124], which may be extended to psychiatric illnesses. In addition, recent optical imaging techniques allow combination of fiber photometry and electrophysiology to study specific cell population activity via genetically encoded calcium indicators, including during deep brain stimulation [125, 126]. Cell-specific viral transfection in calcium imaging or optogenetics helps to study specific ion channels or neurotransmitters. However, the use of viruses in humans is very limited. Further technological advancements are necessary to harness optical methods in the clinical setting.
Currently, the use of DBS in addiction remains off-label, primarily due to the lack of high-quality clinical evidence. As seen in Table 2, most human studies consist of observational studies with a small sample size. However, more clinical trials are currently being conducted. The ClinicalTrials.gov database show that there are currently around 10 DBS trials underway trying to tackle this paucity of scientific evidence, targeting the NAc, ALIC, and STN [127]. As demonstrated in the present review, they are all very promising targets. In addition, we would add PFC to this list given the success from animal studies [47] as well as human imaging studies showing its dysfunction in subjects with substance use disorder [128].
Supporting the potential of DBS in alleviating addiction-related behaviors, DBS to treat obesity has also shown early successes [129,130,131]. Given the similarities between substance use disorders and obesity, the findings in DBS for substance use disorder presented so far may be translatable to DBS for obesity. With the increasing prevalence and comorbidities associated with excessive calorie consumption in the modern society, this can be hugely beneficial.
While it is exciting to perform cutting-edge patient surgeries, it is also important to recognize that DBS is not an immediate cure and requires careful patient selection, consent, and patient-based optimization (including treating other comorbidities); otherwise, DBS can lead to a risk of major adverse outcomes [132]. DBS also has cost, translational, and capacity limitations for a highly prevalent disorder, but has the advantage of elucidating fundamental neural biology capable of detecting new therapeutic targets.
In our literature search, all human studies that involve stimulation of deep structures were conducted exclusively with high-frequency stimulation, as commonly used in DBS for movement disorders (see Table 2). Therefore, future work to compare how stimulation at different frequencies may affect the biomarkers such as LFP in humans would be beneficial.
The duration of trials may also be important. One of the possible reasons attributed to the failure of early trials in DBS for depression is the short treatment duration, as trials with longer duration appeared to show significant efficacy [6, 133,134,135]. DBS duration effects will need to be assessed in larger clinical trials. Further, for neuropsychiatric conditions such as addiction, careful consideration of the ethical aspect is required.
Ethical Considerations
Although DBS is much safer than early forms of psychosurgery such as lobotomy, it is not a risk-free treatment [136]. Therefore, clinicians are obliged to always have the patients’ best interest in their mind. The risks and benefits of the treatment need to be balanced appropriately. One of the main issues with human trials is the principle of autonomy, as patients with addictive disorders may not have the capacity to consent to such process. Furthermore, there are concerns that DBS (and indeed any forms of psychosurgery) may alter a patient’s personality, which can lead to actions and consequences that may otherwise not be performed [137,138,139]. Academic societies and government institutions should also provide a medico-legal framework to ensure both medical professionals and patients are protected. A full ethical discussion is out of the scope of this review. Nevertheless, we recommend that the clinical team be multidisciplinary, with access to the resources and expertise to assess and manage the patients before and after the DBS surgery.
Conclusions
Preclinical and clinical studies suggest DBS is a potential treatment for refractory addictive disorders after other non-invasive options fail. Here, we reviewed these studies and their implications. However, given the uncertainty in its mechanism of action and a lack of large scale randomized controlled trials, clinicians and scientists should be cautiously optimistic. We also provided recommendations on the directions of future research, such as the need to identify reliable biomarkers, for which LFPs in the NAc and in vivo electrochemical measurement of monoamines so far appear the most promising options. With further research, we believe it can prove to be a highly effective treatment for a selected group of patients.
References
Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113(10):1905–26.
U.S. Department of Health and Human Services (HHS) OotSG. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS; 2016. Available from: https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf.
Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–84.
Soyka M, Mutschler J. Treatment-refractory substance use disorder: focus on alcohol, opioids, and cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:148–61.
Edwards CA, Kouzani A, Lee KH, Ross EK. Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin Proc. 2017;92(9):1427–44.
Yuen J, Rusheen AE, Price JB, Barath AS, Shin H, Kouzani AZ, et al. Biomarkers for deep brain stimulation in animal models of depression. Neuromodulation. 2021.
Marceglia S, Rosa M, Servello D, Porta M, Barbieri S, Moro E, et al. Adaptive deep brain stimulation (aDBS) for Tourette syndrome. Brain Sci. 2017;8(1).
Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–93.
Fakhrieh-Asl G, Sadr SS, Karimian SM, Riahi E. Deep brain stimulation of the orbitofrontal cortex prevents the development and reinstatement of morphine place preference. Addiction Biology. 2020;25(4):e12780.
Degoulet M, Tiran-Cappello A, Combrisson E, Baunez C, Pelloux Y. Subthalamic low-frequency oscillations predict vulnerability to cocaine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(14):e2024121118.
Müller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Büntjen L, et al. Nucleus accumbens deep brain stimulation for alcohol addiction - safety and clinical long-term results of a pilot trial. Pharmacopsychiatry. 2016;49(4):170–3.
Chen L, Li N, Ge S, Lozano AM, Lee DJ, Yang C, et al. Long-term results after deep brain stimulation of nucleus accumbens and the anterior limb of the internal capsule for preventing heroin relapse: an open-label pilot study. Brain Stimul. 2019;12(1):175–83.
Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162(4):712–25.
Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(1–2):75–114.
Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67(1):176–97.
Muller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res. 2015;277:146–92.
Li Y, Simmler LD, Van Zessen R, Flakowski J, Wan JX, Deng F, et al. Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science. 2021;373(6560):1252–6.
Samaha AN, Khoo SY, Ferrario CR, Robinson TE. Dopamine “ups and downs” in addiction revisited. Trends Neurosci. 2021;44(7):516–26.
McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RAM, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8(6):1857–69.
Nakamura K. The role of the dorsal raphe nucleus in reward-seeking behavior. Front Integr Neurosci. 2013;7:60.
Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59(3):486–96.
Luscher C, Janak PH. Consolidating the circuit model for addiction. Annu Rev Neurosci. 2021;44:173–95.
Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61(5):641–4.
Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345(6204):1616–20.
Brown RML, A. J.; Kim, J. H. The role of mitogen-activated protein kinase in treatment strategies for fear and drug addiction. Advances in Protein Kinases. London: IntechOpen; 2012.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–85.
Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20.
Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14(2):342–52.
Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102(2):491–6.
Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–51.
Iino Y, Sawada T, Yamaguchi K, Tajiri M, Ishii S, Kasai H, et al. Dopamine D2 receptors in discrimination learning and spine enlargement. Nature. 2020;579(7800):555–60.
Luscher C, Pascoli V. “Ups, downs, and sideways” of dopamine in drug addiction. Trends Neurosci. 2021;44(8):593–4.
Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347(6222):659–64.
Wilden JA, Qing KY, Hauser SR, McBride WJ, Irazoqui PP, Rodd ZA. Reduced ethanol consumption by alcohol-preferring (P) rats following pharmacological silencing and deep brain stimulation of the nucleus accumbens shell. J Neurosurg. 2014;120(4):997–1005.
Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27(51):14179–89.
Niu L, Guo Y, Lin Z, Shi Z, Bian T, Qi L, et al. Noninvasive ultrasound deep brain stimulation of nucleus accumbens induces behavioral avoidance. Science China Life sciences. 2020;63(9):1–9.
Wade CL, Kallupi M, Hernandez DO, Breysse E, de Guglielmo G, Crawford E, et al. High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacology. 2017;42(9):1850–9.
OSF Home. Deep brain stimulation for addiction - a scoping review and recommendations for future research: Center for Open Science; 2021 [8/29/21]. Available from: https://osf.io/pqyzn/?view_only=d6ccd897bc76442b8eace09025451c0f.
Liu H-Y, Jin J, Tang J-S, Sun W-X, Jia H, Yang X-P, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13(1):40–6.
Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28(35):8735–9.
Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, et al. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Depend. 2013;129(1–2):70–81.
Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci. 2013;33(36):14446–54.
Hamilton J, Lee J, Canales JJ. Chronic unilateral stimulation of the nucleus accumbens at high or low frequencies attenuates relapse to cocaine seeking in an animal model. Brain Stimul. 2015;8(1):57–63.
Hadar R, Vengeliene V, Barroeta Hlusicke E, Canals S, Noori HR, Wieske F, et al. Paradoxical augmented relapse in alcohol-dependent rats during deep-brain stimulation in the nucleus accumbens. Transl Psychiatry Psychiatry. 2016;6(6):e840.
Batra V, Tran TLN, Caputo J, Guerin GF, Goeders NE, Wilden J. Intermittent bilateral deep brain stimulation of the nucleus accumbens shell reduces intravenous methamphetamine intake and seeking in Wistar rats. J Neurosurg. 2017;126(4):1339–50.
Mehdipour S, Alaei HA, Pilehvariyan AA. The effect of medial prefrontal cortex electrical stimulation on passive avoidance memory in healthy and addict rats. Adv. 2015;4:254.
Guercio LA, Wimmer ME, Swinford-Jackson SE, Pierce RC, Vassoler FM, Schmidt HD. Deep brain stimulation of the infralimbic cortex attenuates cocaine priming-induced reinstatement of drug seeking. Brain Research. 2020;1746:147011.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68(3):816–71.
Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review. Stereotact Funct Neurosurg. 2015;93(2):75–93.
Gibson GD, Millan EZ, McNally GP. The nucleus accumbens shell in reinstatement and extinction of drug seeking. Eur J Neurosci. 2019;50(3):2014–22.
Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2009;92(3):474–9.
Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29(2):E12.
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, et al. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38(4):690–8.
Beaulieu CL, Thorn BE. Focal brain stimulation attenuates morphine withdrawal behaviors. Behav Neurosci. 1986;100(4):504–11.
Ma Y, Chen N, Wang HM, Meng FG, Zhang JG. Inhibition of the reinstatement of morphine-induced place preference in rats by high-frequency stimulation of the bilateral nucleus accumbens. Chin Med J (Engl). 2013;126(10):1939–43.
Martínez-Rivera FJ, Rodriguez-Romaguera J, Lloret-Torres ME, Do Monte FH, Quirk GJ, Barreto-Estrada JL. Bidirectional modulation of extinction of drug seeking by deep brain stimulation of the ventral striatum. Biol Psychiatry. 2016;80(9):682–90.
Chang H, Gao C, Sun K, Xiao L, Li X, Jiang S, et al. Continuous high frequency deep brain stimulation of the rat anterior insula attenuates the relapse post withdrawal and strengthens the extinction of morphine seeking. Frontiers in Psychiatry. 2020;11 (no pagination).
Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, et al. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59(6):452–9.
Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A. 2010;107(3):1196–200.
Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry. 2009;80(6):659–66.
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999;11(1):71–8.
Wong JE, Cao J, Dorris DM, Meitzen J. Genetic sex and the volumes of the caudate-putamen, nucleus accumbens core and shell: original data and a review. Brain Struct Funct. 2016;221(8):4257–67.
Xia X, Fan L, Cheng C, Eickhoff SB, Chen J, Li H, et al. Multimodal connectivity-based parcellation reveals a shell-core dichotomy of the human nucleus accumbens. Hum Brain Mapp. 2017;38(8):3878–98.
Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, et al. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci. 2013;33(41):16383–93.
Budilin SY, Midzyanovskaya IS, Shchegolevskii NV, Ioffe ME, Bazyan AS. Asymmetry in dopamine levels in the nucleus accumbens and motor preference in rats. Neurosci Behav Physiol. 2008;38(9):991–4.
Navarro PA, Lopez WOC, Paranhos T, Lovo E, De Oliveira-Souza R, Gorgulho AA, et al. Safety and feasibility of nucleus accumbens surgery for drug addiction: a systematic review. Neuromodulation. 2021.
Kuhn J, Moller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TOJ, et al. Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry. 2014;19(2):145–6.
Zhu R, Zhang Y, Wang T, Wei H, Zhang C, Li D, et al. Deep brain stimulation of nucleus accumbens with anterior capsulotomy for drug addiction: a case report. Stereotact Funct Neurosurg. 2020;98(5):345–9.
Hill DF, Parent KL, Atcherley CW, Cowen SL, Heien ML. Differential release of dopamine in the nucleus accumbens evoked by low-versus high-frequency medial prefrontal cortex stimulation. Brain Stimul. 2018;11(2):426–34.
Zbukvic IC, Kim JH. Divergent prefrontal dopaminergic mechanisms mediate drug- and fear-associated cue extinction during adolescence versus adulthood. Eur Neuropsychopharmacol. 2018;28(1):1–12.
Lupi M, Sepede G, Cinosi E, Martinotti G, di Giannantonio M. The efficacy of transcranical direct current stimulation in pregabalin abuse: a case report. Journal of ECT. 2018;34(1):e14–5.
Perini I, Kampe R, Arlestig T, Karlsson H, Lofberg A, Pietrzak M, et al. Repetitive transcranial magnetic stimulation targeting the insular cortex for reduction of heavy drinking in treatment-seeking alcohol-dependent subjects: a randomized controlled trial. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2020;45(5):842–50.
Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298(5601):2209–11.
Schellekens AF, de Bruijn ER, van Lankveld CA, Hulstijn W, Buitelaar JK, de Jong CA, et al. Alcohol dependence and anxiety increase error-related brain activity. Addiction. 2010;105(11):1928–34.
Leong SL, Glue P, Manning P, Vanneste S, Lim LJ, Mohan A, et al. Anterior cingulate cortex implants for alcohol addiction: a feasibility study. Neurotherapeutics. 2020;17(3):1287–99.
De Ridder D, Vanneste S, Kovacs S, Sunaert S, Dom G. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study. Neurosci Lett. 2011;496(1):5–10.
De Ridder D, Leong SL, Manning P, Vanneste S, Glue P. Anterior cingulate implant for obsessive-compulsive disorder. World Neurosurgery. 2017;97:754.e7-.e16.
Kuhn J, Gründler TO, Bauer R, Huff W, Fischer AG, Lenartz D, et al. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16(4):620–3.
Ibrahim C, Rubin-Kahana DS, Pushparaj A, Musiol M, Blumberger DM, Daskalakis ZJ, et al. The insula: a brain stimulation target for the treatment of addiction. Front Pharmacol. 2019;10:720.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–88.
Witjas T, Baunez C, Henry JM, Delfini M, Regis J, Cherif AA, et al. Addiction in Parkinson’s disease: impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20(8):1052–5.
Eusebio A, Witjas T, Cohen J, Fluchere F, Jouve E, Regis J, et al. Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84(8):868–74.
Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and “natural” rewards. Nat Neurosci. 2005;8(4):484–9.
Pinel JP, Van Oot PH, Mucha RF. Intensification of the alcohol withdrawal syndrome by repeated brain stimulation. Nature. 1975;254(5500):510–1.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–60.
McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27(46):12601–10.
Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–40.
Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168(4):850–62.
Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biol Psychiat. 2011;69(11):e41–2.
Zhang C, Li J, Li D, Sun B. Deep brain stimulation removal after successful treatment for heroin addiction. Aust N Z J Psychiatry. 2020;54(5):543–4.
Heldmann M, Berding G, Voges J, Bogerts B, Galazky I, Müller U, et al. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS One. 2012;7(5):e36572.
Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, et al. Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res. 2009;15(4):196–201.
Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66(1):E218; discussion E.
Lupi M, Martinotti G, Santacroce R, Cinosi E, Carlucci M, Marini S, et al. Transcranial direct current stimulation in substance use disorders: a systematic review of scientific literature. Journal of ECT. 2017;33(3):203–9.
Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev. 2019;104:118–40.
Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18(11):685–93.
Hone-Blanchet A, Ciraulo DA, Pascual-Leone A, Fecteau S. Noninvasive brain stimulation to suppress craving in substance use disorders: review of human evidence and methodological considerations for future work. Neurosci Biobehav Rev. 2015;59:184–200.
Ward HB, Mosquera MJ, Suzuki J, Mariano TY. A systematic review of noninvasive brain stimulation for opioid use disorder. Neuromodulation. 2020;23(3):301–11.
Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2472–80.
Abbott. Abbott introduces neurosphere™ virtual clinic, first-of-its-kind remote neuromodulation patient-care technology in the U.S. 2021 [Available from: https://abbott.mediaroom.com/2021-03-08-Abbott-Introduces-NeuroSphere-TM-Virtual-Clinic-First-of-its-Kind-Remote-Neuromodulation-Patient-Care-Technology-in-the-U-S.
Boutet A, Ranjan M, Zhong J, Germann J, Xu D, Schwartz ML, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405–14.
Davidson B, Hamani C, Huang Y, Jones RM, Meng Y, Giacobbe P, et al. Magnetic resonance-guided focused ultrasound capsulotomy for treatment-resistant psychiatric disorders. Oper Neurosurg (Hagerstown). 2020;19(6):741–9.
Gao G, Wang X, He S, Li W, Wang Q, Liang Q, et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Stereotact Funct Neurosurg. 2003;81(1–4):96–104.
Hariz MI, Shamsgovara P, Johansson F, Hariz G, Fodstad H. Tolerance and tremor rebound following long-term chronic thalamic stimulation for Parkinsonian and essential tremor. Stereotact Funct Neurosurg. 1999;72(2–4):208–18.
McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29(16):5354–63.
Ge S, Geng X, Wang X, Li N, Chen L, Zhang X, et al. Oscillatory local field potentials of the nucleus accumbens and the anterior limb of the internal capsule in heroin addicts. Clin Neurophysiol. 2018;129(6):1242–53.
Stenner MP, Durschmid S, Rutledge RB, Zaehle T, Schmitt FC, Kaufmann J, et al. Perimovement decrease of alpha/beta oscillations in the human nucleus accumbens. J Neurophysiol. 2016;116(4):1663–72.
Miller KJ, Prieto T, Williams NR, Halpern CH. Case studies in neuroscience: the electrophysiology of a human obsession in nucleus accumbens. J Neurophysiol. 2019;121(6):2336–40.
Medtronic. PERCEPT PC NEUROSTIMULATOR 2020 [Available from: https://www.medtronic.com/uk-en/patients/treatments-therapies/deep-brain-stimulation-parkinsons-disease/why-choose-medtronic/our-medtronic-dbs-system.html.
U.S. National Library of Medicine. Brain electrophysiological study(EEG/ERP) on opiate addicts treating by bilateral NAc/ALIC deep brain stimulation 2021 [6/6/21]. Available from: https://clinicaltrials.gov/ct2/show/NCT02594306?term=deep+brain+stimulation&cond=Addiction&draw=2&rank=13.
Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009;Chapter 7:Unit7 1.
Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49(10):1763–73.
Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76(19):5697–704.
Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM, Wightman RM. Hitchhiker’s guide to voltammetry: acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem Neurosci. 2017;8:221−34.
Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7(2):126–9.
Yuen J, Goyal A, Rusheen AE, Kouzani AZ, Berk M, Kim JH, et al. Cocaine increases stimulation-evoked serotonin efflux in the nucleus accumbens. J Neurophysiol. 2022.
Yuen J, Goyal A, Rusheen AE, Kouzani AZ, Berk M, Kim JH, et al. Cocaine-induced changes in tonic dopamine concentrations measured using multiple-cyclic square wave voltammetry in vivo. Frontiers in Pharmacology. 2021;12(1710).
Oh Y, Heien ML, Park C, Kang YM, Kim J, Boschen SL, et al. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens Bioelectron. 2018;121:174–82.
Atcherley CW, Wood KM, Parent KL, Hashemi P, Heien ML. The coaction of tonic and phasic dopamine dynamics. Chem Commun (Camb). 2015;51(12):2235–8.
Rusheen AE, Gee TA, Jang DP, Blaha CD, Bennet KE, Lee KH, et al. Evaluation of electrochemical methods for tonic dopamine detection in vivo. Trends Analyt Chem. 2020;132.
Shin H, Goyal A, Barnett JH, Rusheen AE, Yuen J, Jha R, et al. Tonic serotonin measurements in vivo using N-shaped multiple cyclic square wave voltammetry. Anal Chem. 2021.
Gittis AH, Yttri EA. Translating insights from optogenetics to therapies for Parkinson’s disease. Curr Opin Biomed Eng. 2018;8:14–9.
Spix TA, Nanivadekar S, Toong N, Kaplow IM, Isett BR, Goksen Y, et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science. 2021;374(6564):201–6.
Schor JS, Montalvo IG, Spratt PWE, Brakaj RJ, Stansil JA, Bender KJ, et al. Therapeutic deep brain stimulation disrupts subthalamic nucleus activity dynamics in Parkinsonian mice (Preprint). bioRxiv. 2021:2021.11.12.468404.
Patel AA, McAlinden N, Mathieson K, Sakata S. Simultaneous electrophysiology and fiber photometry in freely behaving mice. Front Neurosci. 2020;14:148.
U.S. National Library of Medicine. ClinicalTrials.gov 2021 [6/6/21]. Available from: https://clinicaltrials.gov/ct2/home.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–69.
Formolo DA, Gaspar JM, Melo HM, Eichwald T, Zepeda RJ, Latini A, et al. Deep brain stimulation for obesity: a review and future directions. Front Neurosci. 2019;13:323.
Casquero-Veiga M, Bueno-Fernandez C, Romero-Miguel D, Lamanna-Rama N, Nacher J, Desco M, et al. Exploratory study of the long-term footprint of deep brain stimulation on brain metabolism and neuroplasticity in an animal model of obesity. Sci Rep. 2021;11(1):5580.
Halpern CH, Torres N, Hurtig HI, Wolf JA, Stephen J, Oh MY, et al. Expanding applications of deep brain stimulation: a potential therapeutic role in obesity and addiction management. Acta Neurochir (Wien). 2011;153(12):2293–306.
Zhang C, Huang Y, Zheng F, Zeljic K, Pan J, Sun B. Death from opioid overdose after deep brain stimulation: a case report. Biol Psychiatry. 2018;83(1):e9–10.
Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240–8.
Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, et al. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am J Psychiatry. 2019;176(11):949–56.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839–49.
Caruso JP, Sheehan JP. Psychosurgery, ethics, and media: a history of Walter Freeman and the lobotomy. Neurosurg Focus. 2017;43(3):E6.
Freeman W. Ethics of psychosurgery. N Engl J Med. 1953;249(20):798–801.
Ali R, DiFrancesco MF, Ho AL, Kampman KM, Caplan AL, Halpern CH. Attitudes toward treating addiction with deep brain stimulation. Brain Stimul. 2016;9(3):466–8.
Trujols J, Manresa MJ, Batlle F, Duran-Sindreu S, Pérez de Los Cobos J. Deep brain stimulation for addiction treatment: further considerations on scientific and ethical issues. Brain Stimul. 2016;9(5):788–9.
Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363–71.
Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(10):1152–3.
Valencia-Alfonso CE, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, et al. Effective deep brain stimulation in heroin addiction: a case report with complementary intracranial electroencephalogram. Biol Psychiatry. 2012;71(8):e35–7.
Voges J, Muller U, Bogerts B, Munte T, Heinze H-J. Deep brain stimulation surgery foralcohol addiction. World Neurosurgery. 2013;80(3-4):S28.e1–31.
Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M, et al. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42(6):288–91.
Heinze HJ, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf JM, et al. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci. 2009;3:22.
Gonçalves-Ferreira A, do Couto FS, Rainha Campos A, Lucas Neto LP, Gonçalves-Ferreira D, Teixeira J. Deep Brain Stimulation for Refractory Cocaine Dependence. Biol Psychiatry. 2016;79(11):e87–9.
Ge S, Chen Y, Li N, Qu L, Li Y, Jing J, et al. Deep Brain Stimulation of Nucleus Accumbens for Methamphetamine Addiction: Two Case Reports. World Neurosurg. 2019;122:512–7.
Sildatke E, Schuller T, Huys D, Grundler TOJ, Ullsperger M, Visser-Vandewalle V, et al. Error-Related Activity in Striatal Local Field Potentials and Medial Frontal Cortex: Evidence From Patients With Severe Opioid Abuse Disorder. Frontiers in Human Neuroscience. 2020;14:627564.
Mahoney JJ, Haut MW, Hodder SL, Zheng W, Lander LR, Berry JH, et al. Deep brain stimulation of the nucleus accumbens/ventral capsule for severe and intractable opioid and benzodiazepine use disorder. Experimental and clinical psychopharmacology. 2021;29(2):210–5.
Acknowledgements
We would like to acknowledge Ms. Leslie Hassett, Outreach Librarian, Mayo Clinic, for her contribution to the thorough literature search. M. B. is supported by a NHMRC Senior Principal Research Fellowship (1156072).
Required Author Forms
23 provided by the authors are available with the online version of this article.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by the NIH (R01NS112176) and the Minnesota Partnership for Biotechnology and Medical Genomics (MNP #19.13).
Author information
Authors and Affiliations
Contributions
K. H. L., Y. O., J. H. K., and J. Y. conceptualized the study. J. Y. collected the data. J. Y. drafted the first manuscript. A. Z. K., M. B., J. H. K., S. T., C. D. B., K. E. B., K. H. L., and Y. O. critically reviewed and revised the manuscript. K. H. L., M. B., J. H. K., H. S., and Y. O. supervised all aspects of this work. J. Y. drafted the figures. All authors accepted the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuen, J., Kouzani, A.Z., Berk, M. et al. Deep Brain Stimulation for Addictive Disorders—Where Are We Now?. Neurotherapeutics 19, 1193–1215 (2022). https://doi.org/10.1007/s13311-022-01229-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01229-4