Abstract
Spinal cord injury (SCI) leads to severe impairment in cardiovascular control, commonly manifested as a rapid, uncontrolled rise in blood pressure triggered by peripheral stimuli—a condition called autonomic dysreflexia. The objective was to demonstrate the translational potential of noninvasive transcutaneous stimulation (TCS) in mitigating autonomic dysreflexia following SCI, using pre-clinical evidence and a clinical case report. In rats with SCI, we show that TCS not only prevents the instigation of autonomic dysreflexia, but also mitigates its severity when delivered during an already-triggered episode. Furthermore, when TCS was delivered as a multisession therapy for 6 weeks post-SCI, the severity of autonomic dysreflexia was significantly reduced when tested in the absence of concurrent TCS. This treatment effect persisted for at least 1 week after the end of therapy. More importantly, we demonstrate the clinical applicability of TCS in treatment of autonomic dysreflexia in an individual with cervical, motor-complete, chronic SCI. We anticipate that TCS will offer significant therapeutic advantages, such as obviating the need for surgery resulting in reduced risk and medical expenses. Furthermore, this study provides a framework for testing the potential of TCS in improving recovery of other autonomic functions such lower urinary tract, bowel, and sexual dysfunction following SCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) disrupts crucial communication between spinal and supraspinal autonomic neural circuits, resulting in severely impaired cardiovascular control [1]. Cardiovascular dysfunction affects the majority of individuals with cervical or high-thoracic SCI (≥ T6 spinal segment) and commonly manifests as autonomic dysreflexia, a condition where systolic blood pressure (BP) can abruptly rise often reaching beyond 300 mmHg [2]. Autonomic dysreflexia is commonly instigated by stimuli from activities of daily living (e.g., bowel routine) [3] or iatrogenic medical procedures (e.g., urodynamic evaluations) [4]. These episodes negatively impact the quality of life, interfere with clinical procedures, and can be life-threatening emergencies [5]. Alarmingly, the sporadic episodes of autonomic dysreflexia can occur more than 40 times/day [6] and can lead to myocardial infarction [7] and stroke [8]. Overall, cardiovascular complications are among the leading causes of morbidity and mortality following SCI [9] and regaining cardiovascular control is an urgent unmet need in this population [10].
Despite the detrimental consequences, recovery of cardiovascular function following SCI remains an underexplored research avenue when compared with motor function. Moreover, the majority of pre-clinical interventions have failed to demonstrate clinical efficacy [11]. Furthermore, recovery becomes even more challenging in individuals with a more refractory, chronic SCI [12].
The ability of electrical interfaces to activate disconnected neuronal circuits has gained overwhelming attention in recent years. Epidural stimulation of the injured spinal cord has shown restoration of voluntary motor control in completely paralyzed humans [13] as well as in rodent models [14]. Similarly, improvements in cardiovascular function following SCI have also been reported with epidural stimulation [15,16,17]. While promising, a major drawback of epidural stimulation is the need for an invasive and expensive surgical procedure. Furthermore, the inability to re-position an implanted electrode considerably limits the adaptability of this procedure across various spinal levels to target specific functions.
We have demonstrated that non-invasive transcutaneous stimulation (TCS) of the spinal cord can ameliorate orthostatic hypotension in individuals with SCI [18]. However, whether TCS can mitigate autonomic dysreflexia episodes remains to be tested. Here, we used a translational approach to examine the efficacy of TCS in a rat model of chronic SCI and to validate its clinical feasibility in an individual with SCI. We hypothesized that acute TCS will prevent the instigation of autonomic dysreflexia despite the noxious trigger being present and will also interrupt autonomic dysreflexia when initiated during an ongoing hypertensive episode. Furthermore, we tested whether long-term TCS mitigates autonomic dysreflexia, when tested independently of active TCS. Finally, we examined the clinical adaptability of TCS as an acute therapeutic intervention for autonomic dysreflexia in an individual with chronic, motor-complete cervical SCI.
Methods
Pre-clinical Rat Model
The procedures were performed in compliance with the Canadian Council on Animal Care guidelines, with protocols approved by the Animal Care Committee at the University of British Columbia (approval certificate: A18-0183). Forty-three adult (~ 300 g), male Wistar rats (Harlan Laboratories, USA) received a complete transection at the T3 spinal segment and were divided across four groups (Fig. 1). Group 1 (n = 13) was utilized for acute TCS experiments and groups 2, 3, and 4 (n = 10/group) were used in the long-term TCS experiments.
Overview of the translational approach. Upper panel represents individual timelines for animal experiments showing four animal groups. A schematic drawing of a rat shows the experimental setup of cathode and anode for TCS, a temperature probe, and a balloon-tipped catheter for CRD. Black hearts represent wireless cardiovascular assessments. Lower panel represents the clinical case. A T2 magnetic resonance imaging in the sagittal plane demonstrates the SCI at C4 level. A timeline shows the TCS experiments delivered at 3 years post-SCI. A schematic drawing shows the experimental setup for a stimulating cathode with a skin temperature probe and two anodes. Please refer to main text for additional details
SCI Procedure
A laminectomy was performed to expose the T3 spinal segment. Dura mater was incised and the spinal cord was completely transected using micro-scissors [19]. Dura mater was closed using 9-0 sutures, followed by closure of muscle and skin using 4-0 sutures [20]. Pre- and post-operative care was performed as described previously [21].
Telemeter Implantation
Six weeks post-SCI, rats were implanted with a wireless telemeter (TRM54P; Kaha Sciences, New Zealand). With the rat placed in a supine position, an inguinal skin incision was made to expose the femoral artery. The pressure-sensing tip of the telemeter was inserted and secured in the femoral artery, followed by skin closure using 4-0 sutures.
Experimental Autonomic Dysreflexia
Autonomic dysreflexia was induced via colorectal distension (CRD) using a balloon-tipped catheter (AA6110-Coloplast, Canada). Beat-by-beat BP and heart rate (HR) were recorded using an analog-to-digital converter (PowerLab/16SP ML 795, LabChart 8, ADInstruments, USA). The balloon was inflated with 2 mL of air over 10 s, remaining inflated for 60 s. The difference of systolic and diastolic BP (SBP and DBP) and HR between baseline and the peak value during distension was calculated and averaged across two trials per rat.
Acute (Short-Term) TCS
Group 1 rats received no TCS until 6 weeks after SCI when the acute, real-time effect of TCS was tested. Stimulations (1 ms, 30 Hz, biphasic pulses, 5–10 mA) were delivered using a Model 2100 Isolated Pulse Stimulator (A-M systems) via cathode (1 cm in diameter; DBF3D77, Biofeedback Resources International, USA) placed at the mid-dorsal surface at the T7 level. Anode (Kendall™ ER88007, McKesson, Canada) was placed ventrally along the femur (Fig. 1). The efficacy of acute TCS was tested across two distinct therapeutic principles. First, the ability of TCS to prevent autonomic dysreflexia was examined by initiating TCS 2–3 min prior to CRD. Second, the ability of TCS to interrupt autonomic dysreflexia was tested by initiating TCS 15 s after achieving full distension.
Chronic (Long-Term) TCS
TCS was delivered as a multi-session therapy starting at 5 days post-SCI and continuing for 6 weeks. Stimulations were delivered for 30 min (10 mA, 30 Hz) as two 15-min periods separated by 5 min. Rats were randomly allocated to either alternate day TCS (3 days/week; group 2) or daily TCS (5 days/week; group 3) or no TCS (group 4) groups. One rat died post-surgery, resulting in n = 9 for group 4. In order to test the potentially permanent effects of TCS, cardiovascular assessments during CRD were performed at 1, 3, and 7 days after the last stimulation session, independent of active, concurrent TCS. All assessments were performed with personnel blinded to the animal group allocation. Arrhythmia events were manually identified on arterial pressure waveforms. The proportion of rats with arrhythmia and the event frequency (events/CRD episode) were quantified. Local skin temperature and core body temperature were recorded via a skin probe (MLT422, AD Instruments) and a rectal thermometer (20250-91, Cole-Parmer, Canada), respectively.
Statistical Analyses
Baseline hemodynamics at rest and autonomic dysreflexia severity within acute TCS group were compared via two-tailed, paired t tests. Baseline hemodynamics and autonomic dysreflexia severity between chronic TCS groups was compared via one-way ANOVA (repeated measures were used for comparison within a group at different timepoints), followed by Tukey’s test for multiple comparisons. Proportion of rats with arrhythmia events was compared using the Fisher’s exact test, followed by Pearson chi-square test for pairwise comparisons. Frequency of arrhythmia events were compared across groups using the Kruskal-Wallis test, followed by Dunn’s test for multiple comparisons. Skin and rectal temperatures were compared using one-way repeated measures ANOVA. Data are reported as mean ± standard deviation (SD) with statistical significance at p < 0.05.
Clinical Translation (Case Report)
Study Design
A 37-year-old man with a chronic (3 years) motor-complete SCI (American Spinal Injury Association Impairment Scale A) at the fourth cervical (C4) level was assessed across three laboratory visits. The participant provided written informed consent. This case study was approved by the University of British Columbia Clinical Ethics Board. The ability of TCS to prevent (5 trials) and interrupt (4 trials) autonomic dysreflexia was tested across visits.
Outcome Measures
Beat-by-beat BP was measured via finger photoplethysmography (Finapres® NOVA, Finapres Medical Systems, The Netherlands) corrected to brachial BP (Dinamap Pro 300V2, General Electric, USA) throughout examinations. HR was recorded via 3-lead electrocardiography (ML132, ADInstruments). Skin temperature beneath the cathode was monitored using a temperature probe (MLT422, AD Instruments). HR, BP, and skin temperature were sampled at 1000 Hz using an analog-to-digital converter.
Clinical Autonomic Dysreflexia
Digital anorectal stimulation (DARS) is a routine procedure to initiate a bowel routine and has previously been employed to trigger controlled elevation in BP [22]. DARS was performed by an experienced clinician (AVK) in accordance with recommendations by Coggrave and Norton [23], while both clinician and the participant remaining blinded to the concurrent change in BP. The participant completed a bowel routine within 24 h prior to assessment. The participant laid on his right side and DARS was delivered via an index finger inserted into the rectum, applying gentle pressure for 30–60 s. Considering participant’s safety, the BP was elevated in a controlled manner with DARS being terminated upon participant’s self-perception of autonomic dysreflexia (i.e., feeling flushed, sweating, goose bumps). Surface electromyography (EMG) electrodes (Trigno, Delsys Inc, USA) were placed perianally to bilaterally record from the pelvic floor muscles (PFM) and monitor the influence of DARS on the perineum. EMG data were recorded at 2000 Hz and analyzed offline using custom MATLAB routines (Mathworks, USA). Data were band-stop filtered at 30, 60, 90, and 120 Hz and low-pass filtered at 100 Hz using an 8th order Butterworth filter.
Acute TCS
TCS was delivered as continuous pulses (2 ms, 30 Hz, 20–30 mA) using an isolated bipolar constant current stimulator (Digitimer DS5, Digitimer Ltd., UK). Current was applied at the T7/8 spinous processes using a circular cathode with a diameter of 30 mm (Canadian Medical Products Ltd., Canada) placed on the skin (Fig. 1). Two 5 × 9 cm anodes (Canadian Medical Products Ltd., Canada) were placed on the skin over iliac crests. To determine the ability of TCS to prevent an episode of autonomic dysreflexia, TCS was applied prior to DARS. Alternatively, to determine the ability of TCS to interrupt an episode of autonomic dysreflexia, TCS was initiated 30 s into the DARS, which remained ongoing for an additional 30 s.
Statistical Analyses
The magnitude of the change in hemodynamic responses to DARS was calculated as the difference between the average 1 min baseline and the peak value obtained during DARS. Values are displayed as median Δ change and range. Percentage change was calculated to contextualize the magnitude of the differences between TCS OFF and TCS ON.
Results
Pre-clinical Study
Effect of Acute TCS on Resting Hemodynamics
In order to test whether acute TCS affects resting hemodynamics, beat-by-beat BP and HR were measured 2 min prior to and 2 min after cessation of TCS. HR was elevated by 12 bpm for 2 min after cessation of TCS (p = 0.02). There was no lasting effect of acute TCS on BP (Table 1).
Acute Prevention of Autonomic Dysreflexia
In group 1, CRD induced severe autonomic dysreflexia (ΔSBP 62 ± 15 mmHg; Fig. 2a, c, d). However, when TCS was initiated prior to CRD, the same visceral stimulus resulted in a significantly smaller rise in SBP (14 ± 11 mmHg; p < 0.0001; Fig. 2b–d). Similarly, there was a preventative effect of TCS on the increase in DBP (44 ± 10 vs. 11 ± 7 mmHg; p < 0.0001; Fig. 2e, f) and the concomitant decrease in HR (− 91 ± 69 vs. − 13 ± 11 bpm; p = 0.0026; Fig. 2g, h).
Acute TCS prevents autonomic dysreflexia in rats. a Representative manifestation of an episode of autonomic dysreflexia in one rat with chronic SCI (BP-gray, HR-blue) using CRD via a balloon-tipped catheter (1-balloon inflation begins, 2-balloon fully inflates, and 3-balloon is deflated). b Response to CRD in the same rat while continuous TCS is turned ON prior to balloon inflation shows prevention of autonomic dysreflexia. c, e, and g Time-locked averages of SBP, DBP, and HR, respectively, during CRD in thirteen rats. d, f, and h Quantification of CRD-dependent maximal change in SBP, DBP, and HR from baseline when TCS is OFF vs ON. Dashed line at 20 mmHg in ΔSBP graph (d) represents the clinical definition of autonomic dysreflexia, i.e., rise in SBP by 20 mmHg in response to a peripheral stimulus
Interestingly, when TCS was off, the insertion of a deflated balloon-tipped catheter into the rectum also caused elevation in resting BP (Fig. 2c, e) and concomitant reduction in HR (Fig. 2g). This cardiovascular response to catheter insertion was also prevented by TCS (TCS off vs. on: SBP 134 ± 12 vs. 101 ± 12 mmHg; p < 0.0001, DBP 88 ± 8 vs. 67 ± 9 mmHg; p < 0.0001, HR 465 ± 55 vs. 529 ± 55 bpm; p = 0.0018).
Acute Interruption of Autonomic Dysreflexia
The efficacy of acute TCS to interrupt autonomic dysreflexia was tested by initiating TCS during ongoing CRD in group 1 (Fig. 3a). CRD triggered a rapid increase in SBP (49 ± 21 mmHg) and DBP (34 ± 11 mmHg) with a concomitant decrease in HR (− 42 ± 33 bpm). However, the initiation of TCS immediately reduced the severity of these changes (i.e., ΔSBP 21 ± 12 mmHg, p = 0.0002; Fig. 3b, c, ΔDBP 17 ± 11 mmHg, p < 0.0001; Fig. 3d, e and ΔHR 0.4 ± 30 bpm, p = 0.0029; Fig. 3f, g), despite CRD being maintained.
Acute TCS interrupts autonomic dysreflexia in rats. a Representative manifestation of an episode of autonomic dysreflexia in one rat with chronic SCI (BP-gray, HR-blue) using CRD being interrupted by TCS (1-balloon inflation begins, 2-balloon fully inflates, lightning bolt-TCS initiated, and 3-balloon is deflated). b, d, and f Time-locked averages of SBP, DBP, and HR, respectively, during similar experiment in thirteen rats. c, e, and g Quantification of CRD-dependent maximal change in SBP, DBP, and HR from baseline when TCS is OFF vs ON. Dashed line at 20 mmHg in ΔSBP graph (c) represents the clinical definition of autonomic dysreflexia, i.e., rise in SBP by 20 mmHg in response to a peripheral stimulus
Effect of Chronic TCS on Resting Hemodynamics
Beat-by-beat BP and HR were measured at three timepoints after the cessation of 6-week alt. day or daily TCS. The resting hemodynamics remained largely unaffected by long-term spinal cord stimulations (Table 2). Although one-way repeated measures ANOVA revealed statistically significant effect in DBP, MAP, and HR within alt. day TCS group and HR in daily TCS group, post hoc pairwise comparisons between timepoints showed no statistically significant effect. Similarly, one-way ANOVA showed a significant difference in resting SBP and MAP between the groups on day 3 after the cessation of TCS, but pairwise post hoc comparisons revealed no significant differences. This suggests that the resting hemodynamics remain largely unaltered in response to long-term TCS.
Chronic Treatment of Autonomic Dysreflexia
Cardiovascular assessments were performed on 1, 3, and 7 days following cessation of TCS (Fig. 4a–i). Group comparisons revealed significant differences in CRD-induced rise in SBP (day 1, p < 0.0001; day 3, p = 0.0003; and day 7, p = 0.0002; Fig. 4a–c) and DBP (day 1, p = 0.0019; day 3, p = 0.008; and day 7, p = 0.014; Fig. 4d–f). Similar comparisons revealed a significant difference in decease in HR at days 3 and 7 but not on day 1 (day 1, p = 0.10; day 3, p = 0.003; and day 7, p = 0.009; Fig. 4g–i). In cases where the group analysis revealed a significant difference, pairwise comparisons revealed that both treatment groups were significantly improved compared with no TCS control (p ≤ 0.017) and that there were no differences between the two treatment groups (p ≥ 0.46).
Effects of chronic multisession TCS on autonomic dysreflexia in rats. a–c Comparison of severity of rise in SBP in response to CRD in groups receiving no TCS, alternate day TCS, and daily TCS at 1, 3, and 7 days post cessation of multi-session TCS therapy. The results show significant effects of TCS on ΔSBP severity regardless of the stimulation paradigm, lasting for 7 days after the end of stimulation. Similar effects were seen in ΔDBP for all three timepoints (d–f) and ΔHR for days 3 and 7 (g–i)
Mitigation of Arrhythmia
Nine out of nine animals in the control group showed cardiac arrhythmia events during autonomic dysreflexia compared with 5/10 animals in each of the treatment groups (p = 0.03; Fig. 5a, b). Pairwise comparisons revealed a greater proportion of rats experiencing arrhythmias in the control group compared with each of the treatment groups (p = 0.013). The frequency of arrhythmia events was also different across groups (p = 0.003; Fig. 5c), with both treatment groups showing significantly lower arrhythmia frequency compared with control (p = 0.01). No difference was seen between the two treatment groups (p = 0.90).
Effects of chronic multisession TCS on cardiac arrhythmia in rats. a Representation of a normal arterial pressure waveform (top) and a waveform showing two arrhythmia events (red arrows, bottom). b Both TCS paradigms result in a significantly smaller proportion of rats experiencing arrhythmia (Ψ represents significant difference from both treatment groups). c Both TCS paradigms resulted in a significantly lower frequency of arrhythmia events during autonomic dysreflexia (Ψ represents significant difference from both treatment groups)
Temperature Changes During TCS
During the 30 min of TCS, the maximum rise in temperature at the site of stimulation was 3.1 °C (35.5 ± 0.5 to 38.6 ± 0.8 °C; p < 0.0001, Fig. 6a) and 1.8 °C (35.2 ± 0.8 to 37.1 ± 0.7 °C; p < 0.0001, Fig. 6b) in the core body temperature.
Clinical Case
Acute Prevention of Autonomic Dysreflexia
DARS resulted in a consistent, controlled rise in BP (ΔSBP 29 ± 5 and ΔDBP 18 ± 3 mmHg) and a reduction in HR (−4 ± 1 bpm), characteristic of autonomic dysreflexia (representative trace presented in Fig. 7a). The small change in HR is likely a result of the controlled rise in BP during DARS as the participant’s comfort and safety was the primary concern. However, when TCS was turned on prior to DARS (representative trace shown in Fig. 7b), the same visceral stimulus resulted in an 82% reduced rise in SBP (5 ± 3 mmHg; Fig. 7c), 65% reduced rise in DBP (6 ± 3 mmHg; Fig. 7d) and 68% lesser reduction in HR (−1 ± 0.5 bpm; Fig. 7e).
Acute TCS can prevent and interrupt the episodes of autonomic dysreflexia in an individual with SCI. a Manifestation of an episode of autonomic dysreflexia in the participant with chronic, motor-complete SCI in response to DARS and in the absence of TCS (1-DARS ON and 2-DARS OFF). b Response to DARS in the same participant in the presence of continuous TCS shows prevention of autonomic dysreflexia. c, d, and e Quantification of DARS-dependent maximal change in SBP, DBP, and HR from baseline when TCS is OFF vs ON. Dashed line at 20 mmHg in ΔSBP graph (c) represents the clinical definition of autonomic dysreflexia, i.e., rise in SBP by 20 mmHg in response to a peripheral stimulus. f DARS triggers severe rise in BP in the absence of TCS. However, elevated BP is normalized when TCS (lightning bolt) is turned on (1-DARS ON and 2-DARS OFF, 3-TCS OFF). g, h, and i Quantification of DARS-dependent maximal change in SBP, DBP, and HR from baseline when TCS is OFF vs ON. EMG traces showing the PFM response to DARS and TCS are shown for each condition (a, c, and f)
Acute Interruption of Autonomic Dysreflexia
The efficacy of acute TCS to interrupt an episode of autonomic dysreflexia was tested by initiating TCS during an episode of DARS (representative trace shown in Fig. 7f). DARS triggered autonomic dysreflexia in a controlled manner with a rise in BP (ΔSBP 18 ± 1 and ΔDBP 13 ± 1 mmHg) and reduction in HR (−2 ± 0.5 bpm). Initiation of TCS during DARS mitigated autonomic dysreflexia by causing a 49% reduction in SBP (9 ± 4 mmHg; Fig. 7g) and 56% reduction in DBP (6 ± 5 mmHg; Fig. 7h). No TCS-dependent change was seen in HR (−3 ± 0 bpm; Fig. 7i).
EMG Findings
The application of DARS resulted in expected reflexive PFM activity. However, the presence of TCS appeared to reduce the level of tonic PFM activity.
Temperature Changes During TCS
No long-term stimulations were delivered to the participant. The duration of continuous TCS ranged between 1 and 4 min. Simultaneous recording of temperature under cathode revealed a maximum rise of 1.3 °C (32.9 to 34.3 °C; data not shown).
Discussion
The present study shows that acute TCS is an effective strategy for mitigating debilitating episodes of autonomic dysreflexia, with potentially permanent effects achieved via long-term stimulation. Interestingly, alternate day TCS demonstrates similar efficacy as daily TCS, which could be clinically meaningful in reducing the required number of participants’ visits. Long-term TCS also ameliorated cardiac arrhythmias, demonstrating a secondary benefit of substantial clinical importance [24]. Finally, we demonstrate the clinical applicability of TCS by demonstrating TCS-dependent prevention/interruption of autonomic dysreflexia in an individual with chronic, cervical, motor-complete SCI. Previous clinical study by our laboratory utilized monophasic electrical pulses to transcutaneously stimulate the spinal cord and increase BP and alleviate orthostatic hypotension (i.e., low BP) in individuals with SCI. While the spinal cord stimulation paradigm utilized in this prior study is believed to excite propriospinal interneurons and sympathetic preganglionic neurons via sensory afferents [18], biphasic square pulses used in the present study appear to inhibit the sympathetic reflex response resulting in autonomic dysreflexia. This is consistent with the work from Richardson and colleagues that used percutaneous epidural spinal cord stimulation to generate low frequency, low voltage, square wave output to produce an ‘electronic central sympathetic inhibitory mechanism’ to reduce spasticity [25] and autonomic dysreflexia in individuals with SCI [26].
Various pre-clinical approaches have been tested to mitigate autonomic dysreflexia. These broadly include (a) reducing aberrant sprouting of nociceptive sensory afferents [27, 28], (b) minimizing secondary damage [29], and (c) restoring supraspinal control [30, 31]. Most of these strategies have been able to reduce the severity of autonomic dysreflexia by 30–50%. To put this in perspective, a much simpler, noninvasive TCS approach was able to reduce the severity of autonomic dysreflexia by 78% in rats and 82% in a human with chronic SCI. Clinically, pharmacological modulation is among the primary options to manage autonomic dysreflexia [32]. However, a major disadvantage is that most drugs require several minutes to become active and exert prolonged cardiovascular effects [33]. Given the rapid appearance of reflex-driven BP changes in autonomic dysreflexia, spinal cord stimulation may offer a more reliable, immediately acting solution.
Spinal cord stimulation, via invasive means, has shown remarkable potential in improving cardiovascular function in individuals with SCI. Most studies have been focused on mitigating hypotension (i.e., low BP at rest and orthostatic intolerance) following SCI [15,16,17], with one prior report showing mitigation of autonomic dysreflexia [26]. However, despite the highest surgical standards, surgical procedures have an inherent risk of complications [34], with a risk of further spinal cord damage [35]. TCS may offer similar neuromodulatory effects, without the need for surgical implants. From the safety standpoint, we tested a remote possibility of skin overheating [36], and TCS appeared safe for the durations tested.
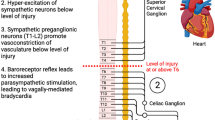
Despite obvious methodological and biophysical differences, both TCS and epidural stimulation share remarkable mechanistic similarities. EMG evidence showing a high degree of congruency in evoked potentials suggests that both methods stimulate common neural correlates [37]. Mechanistically, spinal cord stimulation is derived from the ‘gate control theory’ [38]. At clinically used parameters, electrical stimulation predominantly recruits low-threshold, large-diameter proprioceptive, and cutaneous afferents (Fig. 8) [39]. Computer simulations suggest that during TCS, ~ 8% of the total current flows through the cerebrospinal fluid, generating high current densities along the posterior root afferents [39]. The non-noxious spinal input via large-diameter afferents can transsynaptically activate inhibitory interneurons, thereby ‘closing the gate’ for noxious stimuli (e.g., CRD and DARS) and preventing the uncontrolled reflex response (i.e., autonomic dysreflexia; Fig. 8). While this tends to explain the effects of real-time TCS, the long-lasting effects of TCS are likely a result of the adaptive plasticity of cardiovascular neural circuits—a hypothesis worthy of further comprehensive physiological and neuroanatomical research.
Known mechanisms of autonomic dysreflexia and theoretical framework of how TCS mitigates the dysfunction. Maladaptive small diameter sensory afferents (red) transmit noxious stimuli to ascending propriospinal interneurons (blue). The ascending signal activates thoracolumbar sympathetic preganglionic neurons (SPNs; black) and postganglionic neurons (purple) to trigger vasoconstriction in peripheral vasculature. This uncontrolled hypertensive response remains uninhibited due to the disruption of supraspinal control by SCI (not shown). Application of TCS results in activation of local large-diameter afferents and close the gates for noxious stimulus thereby preventing and/or interrupting the hypertensive response even in the presence of a noxious stimulus
A limitation of this study is the lack of detailed information on the type of cardiac arrhythmia observed during autonomic dysreflexia episodes. Since cardiac arrhythmias were identified in retrospect, ECG recordings were not available in support of the BP data. However, a previous study has shown identical BP traces in rats with SCI in response to CRD and has confirmed arrhythmia as atrioventricular blocks using ECG [40]. Future studies will also examine the minimum stimulation thresholds needed to elicit these therapeutic responses. Another noteworthy observation is that the experimental autonomic dysreflexia in the human participant was conservatively performed in a controlled manner, keeping the individual’s safety in consideration. The efficacy of TCS to mitigate more severe episodes of autonomic dysreflexia is yet to be investigated.
Despite the unanswered questions, electrical neuromodulation is among the most promising treatments for SCI. In addition to highlighting the need for more mechanistic studies, the present study warrants longitudinal clinical investigations for the safety and efficacy of TCS in cardiovascular dysfunction, with possible permeation into the recovery of other crucial autonomic functions, e.g., gastrointestinal and genitourinary systems.
Data Availability
The pertinent data has been carefully documented within this manuscript. Methods described in brief have been referenced to the published studies for further detail. Raw data that is not provided in the article will be made directly available upon request by any qualified investigator.
References
Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol 2009;169:157-164.
Karlsson AK. Autonomic dysreflexia. Spinal Cord 1999;37:383-391.
Kirshblum SC, House JG, O'Connor K C. Silent autonomic dysreflexia during a routine bowel program in persons with traumatic spinal cord injury: a preliminary study. Arch Phys Med Rehabil 2002;83:1774-1776.
Liu N, Zhou MW, Biering-Sorensen F, Krassioukov AV. Cardiovascular response during urodynamics in individuals with spinal cord injury. Spinal Cord 2016.
Wan D, Krassioukov AV. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med 2014;37:2-10.
Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens 2015;28:173-181.
Ho CP, Krassioukov AV. Autonomic dysreflexia and myocardial ischemia. Spinal Cord 2010;48:714-715.
Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Paraplegia 1992;30:355-360.
Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005;43:408-416.
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21:1371-1383.
Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery 2006;59:957–982;discussion 82–7.
Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol 2003;182:247-260.
Rejc E, Angeli CA, Atkinson D, Harkema SJ. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep 2017;7:13476.
Wenger N, Moraud EM, Raspopovic S, et al. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci Transl Med 2014;6:255ra133.
Aslan SC, Legg Ditterline BE, Park MC, et al. Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals With Spinal Cord Injury-Induced Cardiovascular Deficits. Front Physiol 2018;9:565.
West CR, Phillips AA, Squair JW, et al. Association of Epidural Stimulation With Cardiovascular Function in an Individual With Spinal Cord Injury. JAMA Neurol 2018;75:630-632.
Nightingale TE, Walter M, Williams AMM, Lam T, Krassioukov AV. Ergogenic effects of an epidural neuroprosthesis in one individual with spinal cord injury. Neurology 2019;92:338-340.
Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An Autonomic Neuroprosthesis: Noninvasive Electrical Spinal Cord Stimulation Restores Autonomic Cardiovascular Function in Individuals with Spinal Cord Injury. J Neurotrauma 2018;35:446-451.
Sachdeva R, Theisen CC, Ninan V, Twiss JL, Houle JD. Exercise dependent increase in axon regeneration into peripheral nerve grafts by propriospinal but not sensory neurons after spinal cord injury is associated with modulation of regeneration-associated genes. Exp Neurol 2016;276:72-82.
Sachdeva R, Farrell K, McMullen MK, Twiss JL, Houle JD. Dynamic Changes in Local Protein Synthetic Machinery in Regenerating Central Nervous System Axons after Spinal Cord Injury. Neural Plast 2016;2016:4087254.
Ramsey JB, Ramer LM, Inskip JA, Alan N, Ramer MS, Krassioukov AV. Care of rats with complete high-thoracic spinal cord injury. J Neurotrauma 2010;27:1709-1722.
Faaborg PM, Christensen P, Krassioukov A, Laurberg S, Frandsen E, Krogh K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord 2014;52:494-498.
Coggrave MJ, Norton C. The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: a randomized controlled trial of a stepwise protocol. Spinal Cord 2010;48:504-510.
Hector SM, Biering-Sorensen T, Krassioukov A, Biering-Sorensen F. Cardiac arrhythmias associated with spinal cord injury. J Spinal Cord Med 2013;36:591-599.
Richardson RR, McLone DG. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol 1978;9:153-155.
Richardson RR, Cerullo LJ, Meyer PR. Autonomic hyper-reflexia modulated by percutaneous epidural neurostimulation: a preliminary report. Neurosurgery 1979;4:517-520.
Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 1999;19:7405-7414.
Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci 2006;26:2923-2932.
Squair JW, Ruiz I, Phillips AA, et al. Minocycline Reduces the Severity of Autonomic Dysreflexia after Experimental Spinal Cord Injury. J Neurotrauma 2018.
Sachdeva RLJY, Sangha A, Auyeung A, et al. Restoring supraspinal control to promote cardio-autonomic recovery after spinal cord injury. J Neurotrauma 2018;35:A44-A45.
Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci 2013;33:17138-17149.
Krassioukov A, Warburton DE, Teasell R, Eng JJ. Spinal Cord Injury Rehabilitation Evidence Research T. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009;90:682–695.
Ellrodt AG, Ault MJ, Riedinger MS, Murata GH. Efficacy and safety of sublingual nifedipine in hypertensive emergencies. Am J Med 1985;79:19-25.
Takawira N, Han RJ, Nguyen TQ, Gaines JD, Han TH. Spinal cord stimulator and epidural haematoma. Br J Anaesth 2012;109:649-650.
Smith CC, Lin JL, Shokat M, Dosanjh SS, Casthely D. A report of paraparesis following spinal cord stimulator trial, implantation and revision. Pain Physician 2010;13:357-363.
Balmaseda MT, Fatehi MT, Koozekanani SH, Sheppard JS. Burns in functional electric stimulation: two case reports. Arch Phys Med Rehabil 1987;68:452-453.
Hofstoetter US, Freundl B, Binder H, Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PloS One 2018;13:e0192013.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971-979.
Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng 2010;18:637-645.
Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 2002;283:H1734-H1739.
Acknowledgements
Present work is supported by Rick Hansen Foundation, International Collaboration on Repair Discoveries (ICORD), the Blusson Integrated Cures Partnership and Canada Foundation for Innovation (AVK). Dr. Sachdeva is supported by the Craig H. Neilsen Foundation, Canadian Institutes of Health Research, Michael Smith Foundation for Health Research (MSFHR), and Faculty of Medicine, University of British Columbia (Bluma Tischler Fellowship). Dr. Nightingale is supported by MSFHR in collaboration with ICORD. Authors thank Paolo Go (ICORD) for the technical assistance with animal experiments.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. Krassioukov has two pending patent applications: European National Phase Application: Publication No. EP3582850A1, and a patent US National Phase Application: Serial No. US 16/486,788, Apparatus and Methods for Maintaining Physiological Functions. The remaining authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sachdeva, R., Nightingale, T.E., Pawar, K. et al. Noninvasive Neuroprosthesis Promotes Cardiovascular Recovery After Spinal Cord Injury. Neurotherapeutics 18, 1244–1256 (2021). https://doi.org/10.1007/s13311-021-01034-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01034-5