Abstract
Spasticity affects approximately 65% of persons with spinal cord injury (SCI) and negatively impacts function and quality of life. Whole body vibration (WBV) appears to reduce spasticity and improve walking function; however, the optimal dose (frequency/duration) is not known. We compared single-session effects of four different WBV frequency/duration dose conditions on spasticity and walking speed, in preparation for a planned multi-session study. Thirty-five participants with motor-incomplete SCI received four different doses of WBV: high frequency (50 Hz)/short duration (180 s), high frequency/long duration (360 s), low frequency (30 Hz)/short duration, and low frequency/long duration, plus a control intervention consisting of sham electrical stimulation. In all conditions, participants stood on the WBV platform for 45-s bouts with 1 min rest between bouts until the requisite duration was achieved. The frequency/duration dose order was randomized across participants; sessions were separated by at least 1 week. Quadriceps spasticity was measured using the pendulum test at four time points during each session: before, immediately after, 15 min after, and 45 min after WBV. Walking speed was quantified using the 10-m walk test at three time points during each session: baseline, immediately after, and 45 min after WBV. In the full group analysis, no frequency/duration combination was significantly different from the sham-control condition. In participants with more severe spasticity, a greater reduction in stretch reflex excitability was associated with the high frequency/long duration WBV condition. The sham-control condition was associated with effects, indicating that the activity of repeated sitting and standing may have a beneficial influence on spasticity. Trial registration: NCT02340910 (assigned 01/19/2015).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following spinal cord injury (SCI), individuals experience deficits in motor control that affect their function and quality of life. The disruption of descending signals from the brain to the spinal cord creates an imbalance between the excitatory and inhibitory inputs of the spinal cord circuitry that can affect voluntary muscle control and reflex activity [1]. Spasticity is one of the major secondary complications following SCI with an estimated 65% of individuals reporting it [2]. Moreover, 78% of individuals who report spasticity identify that it impacts their quality of life [3]. While spasticity has traditionally been narrowly defined as “a motor disorder characterized by a velocity dependent increase in the tonic stretch reflex with exaggerated tendon jerks…” [4], advances in research and clinical understanding of reflex activity has led to a broadening of this definition to “disordered sensori-motor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” [5]. The dysregulation of reflex activity associated with spasticity can significantly interfere with daily activities [3] such as mobility, positioning, sleep, comfort, and hygiene; in more severe cases, it can lead to muscle contractures that can be painful and disfiguring.
Spasticity is commonly treated with anti-spasmodic medications, such as baclofen or tizanidine. However, a recent survey study of individuals with SCI found that the majority of respondents who take these medications find them ineffective for spasticity management [3]. Furthermore, anti-spasmodic medications often have negative side effects, such as muscle weakness and drowsiness, which can further impede motor function [6]. In recent years, physical therapeutic treatments, such as whole body vibration (WBV) and other forms of afferent stimulation, have gained increasing attention as neuromodulatory approaches with potential to be nonpharmacological alternatives for alleviating spasticity [7,8,9].
With presynaptic inhibitory mechanisms known to be disrupted in persons with spasticity [10,11,12], the hypothesized mechanism of WBV as a therapeutic treatment for spasticity stems from findings that afferent input in the form of vibration activates presynaptic inhibitory that reduce the excitatory influence of Ia afferent input [13]. Prior work from our lab has shown that 12 sessions of WBV (3 days a week for 4 weeks) reduces quadriceps spasticity in persons with incomplete SCI [7]. Additionally, in persons with stroke, 24 sessions of WBV (3 days a week for 8 weeks) were shown to significantly reduce quadriceps spasticity [14]. While this evidence is promising for the use of WBV as a spasticity treatment, a systematic review of the literature found that there is currently no conclusive evidence for its use as an anti-spasmodic treatment in persons with SCI [15]. This is likely due to a lack in understanding of the optimal parameters of WBV for the modulation of spasticity, as well as variability in responsiveness among participants.
Beyond the neuromodulatory effects of vibration on spinal reflex excitability, there is evidence that vibration activates spinal central pattern-generating circuits that underlie the generation of locomotor output [16,17,18]. Early evidence indicates that WBV may improve walking function in persons with SCI [19] and appears to augment the effects of locomotor training in persons with stroke [20]. While WBV is being used in clinical settings to treat individuals with neurologic conditions, there is little evidence available to offer guidance regarding parameters related to frequency and duration of WBV dose.
The purpose of this study was to assess, in participants with chronic SCI, the immediate and delayed single-session effects of four doses of WBV compared to a sham-control intervention. WBV doses were administered as different combinations of high/low frequencies and long/short durations. The primary outcome of interest was change in quadriceps muscle spasticity as measured using the pendulum test. As a secondary outcome, we also assessed the influence of WBV on walking speed. This study of single-session WBV effects was performed with the intent to identify the optimal WBV dose in preparation for a planned multi-session study.
Methods
This study was carried out with approval of the Shepherd Center Research Review Committee. All participants gave written informed consent prior to study enrollment in accordance with the Declaration of Helsinki. This study was registered with clinicaltrials.gov (NCT02340910).
Subjects
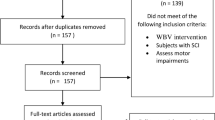
Individuals were eligible for participation if they met the following inclusion criteria: 16–65 years of age with SCI ≥ 6 months duration, ability to stand for at least 1 min using upper extremities for balance only, ability to independently move one leg at least a small amount with or without an assistive device, presence of at least mild spasticity affecting the lower extremity muscles (as determined by participant self-report), and ability to transfer from sit to stand requiring no more than moderate assistance of one person. Individuals with the following exclusion criteria were excluded from participation: neurological level of injury below T12, progressive or potentially progressive spinal lesions (including degenerative or progressive vascular disorders of the spine and/or spinal cord), history of severe or chronic cardiovascular irregularities, difficulty following instructions, and orthopedic problems that would prevent participation in study interventions (i.e., knee or hip flexion contractures > 10°).
Interventions
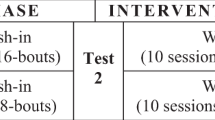
We used a randomized, crossover design consisting of a single session each of four WBV frequency/duration dose combinations and a sham-control (Table 1). Each session was separated by a minimum of 1 week to prevent carryover effects.
Whole Body Vibration (WBV)
Subjects participated in four different WBV frequency/duration dose conditions using a vibration platform (Power Plate Pro5, Performance Health Systems, LLC). During each session, participants stood on the vibration platform with knees slightly flexed (~ 30° from full extension) for 45-s bouts with 1 min of seated rest in-between bouts, as previously described [7]. Two vibration frequencies (low frequency (30 Hz) and high frequency (50 Hz)) and two treatment durations (short duration (180 s, 4 bouts) and long duration (360 s, 8 bouts)) were investigated. These frequencies were selected based on prior studies in participants with stroke and SCI showing effects of WBV on postural control [21] and spasticity [7], respectively. Participants received a single session of each WBV frequency/duration dose condition in a randomized order: low frequency, short duration (LFSD), high frequency, short duration (HFSD), low frequency, long duration (LFLD), and high frequency, long duration (HFLD).
Sham-Control Stimulation
The sham-control was designed to control for effects associated with factors other than vibration, such as the influence of standing with the knees flexed, repeated performance of the sit-to-stand maneuver, or placebo effects. To produce a convincing sham condition, stimulating electrodes were placed in the posterior thoracic region (on the inferior angle of the scapula). The intensity of electrical stimulation was briefly ramped up to a level at which the participants reported perceiving the stimulation, then ramped down and turned off for the remainder of the treatment. During the sham stimulation period, participants stood on the inactive vibration platform for eight 45-s bouts with 1 min of seated rest between bouts, to mimic the activity performed during the real WBV sessions.
Spasticity Assessment
The pendulum test was used to assess stretch-induced quadriceps reflex excitability, which was our primary measure of spasticity. This outcome measure has been used previously as an objective assessment of intervention-related change in spasticity, in studies of both pharmacological [22] and physical therapeutic interventions, such as WBV [7] and transcutaneous spinal cord stimulation [8, 23]. The most spastic lower extremity, as determined by participant self-report during enrollment, was evaluated. For each participant, the same lower extremity was assessed for the duration of the study. Spasticity was assessed at four time points during each session: (1) prior to the start of the intervention (baseline), (2) immediately after the conclusion of the intervention (immediate), (3) 15 min after the conclusion of the intervention (15-min delayed), and (4) 45 min after the conclusion of the intervention (45-min delayed). While the study team requested that participants remain sitting in a semi-reclined position, inactive, during the time between these delayed assessments, participants were permitted to use the restroom as needed. Three pendulum tests were completed at each of the four assessment time points with a minimum of 30 s between each test. Joint kinematics were measured using an electrogoniometer (SG150, Biometrics Ltd., Newport, UK) strapped to the lateral aspect of the knee joint with neoprene wraps. To confirm stretch-induced quadriceps activation during the pendulum test, an electromyographic (EMG) recording electrode (Motion Lab Systems, Baton Rogue, LA) was placed over the rectus femoris muscle. Acquisition and analysis of joint angle and EMG data were conducted using Spike software (Cambridge Electronic Design Limited, Cambridge, England).
The pendulum test was performed with subjects in a semi-reclined position on an adjustable height mat table. Prior work has indicated that similar pendulum test outcomes are produced in individuals with SCI when tested in the fully reclined and semi-reclined positions [24], and the latter position is more comfortable. The test leg was flexed at the knee and hanging over the edge of the mat, the nontest leg was supported on a chair with the knee in extension. The examiner grasped and lifted the heel of the test leg until the knee was fully extended. The heel was then released allowing the lower leg to swing with gravity. This gravity-induced movement of the lower leg stretches the quadriceps muscle evoking a reflex muscle contraction, thus providing a measure of spinal stretch reflex excitability.
The pendulum test parameter of interest for this study was the first swing excursion (FSE) angle of the knee joint. Previous research suggests that FSE is a better indicator of spasticity than other pendulum test components such as the number of oscillations or relaxation index [25]. FSE is the angle at which the movement of the lower leg reverses from flexion to extension after release of the heel. An increase in FSE represents a decrease in spasticity as it indicates a longer length of the quadriceps muscle is attained before a reflex contraction is elicited. For each assessment time point, the mean of three FSEs was calculated for each participant.
Walking Speed
Walking speed was measured at three time points during each session: (1) prior to the start of the intervention (baseline), (2) immediately after intervention (immediate), and (3) 45 min after the conclusion of the intervention (45-min delayed). The study protocol was amended to include the immediate walking assessment after the first 11 participants completed the study; consequently, the first 11 participants did not complete the immediate walk test. During each walking assessment (baseline, immediate, and 45-min delayed), participants completed three 10-m walks at a self-selected speed with a 1 min rest between each walk. Walking speed was recorded during the middle 6 m of the 10-m path using an instrumented walkway (GAITRite, CIR Systems, Franklin, NJ). GAITRite software was used to calculate a weighted average of the walking speed based on the number of footfalls in each of the three walks comprising each assessment.
Data Analysis
Data were analyzed using statistical analysis software (SAS 9.1.2, Cary, NC). Data are presented as the mean ± SEM. Significance was set at α = 0.05 for all analyses. A one-way analysis of variance (ANOVA) was used to test for differences in baseline FSE and baseline walking speed for each condition and also to compare change scores at each time point between the five conditions for both walking speed and FSE. One-tailed paired t tests were used to evaluate within-condition effects comparing baseline FSE values to FSE values at each postintervention time point (immediate, 15-min delayed, and 45-min delayed). One-tailed paired t tests were also used to compare walking speed at baseline to walking speed immediately after intervention and 45 min after intervention. All analyses were completed for the whole group, and for high spasticity and low spasticity subgroups. For subgroup analyses, the median baseline FSE (46.6°) was used to categorize baseline spasticity for each session as high (baseline FSE < 46.6°) or low (baseline FSE > 46.6°). Effect sizes were calculated with the pooled variance for the two values being compared using Cohen’s d and categorized as small (0.2–0.49), moderate (0.5–0.8), or large (> 0.8) as previously described [26]. To account for missing data points, only participants who completed all assessments for a given comparison (i.e., ANOVA, paired t test) were utilized for analysis.
Results
Two-hundred forty-one individuals were assessed for study eligibility. Of those, 35 participants enrolled in the study, and 29 participants completed all sessions (Fig. 1). Participant demographics can be found in Table 2.
Effects of WBV on Stretch-Induced Quadriceps Spasticity
Mean baseline FSE was not different among any of the five intervention sessions (Fig. 2; one-way ANOVA, F = 0.15, p = 0.963). To determine the effect of each WBV dose on quadriceps spasticity, we first compared the mean FSE for each of the four WBV frequency/duration conditions (HFSD, LFSD, HFLD, LFLD) to the sham-control condition. When the full sample was considered, the change in FSE for each of the four WBV frequency/duration dose conditions was not different from sham-control at any of the three postintervention assessments (immediate (F = 0.64, p = 0.63), 15-min delayed (F = 0.44, p = 0.78), and 45-min delayed (F = 0.44, p = 0.78). When evaluating within-condition effects, the sham-control condition was associated with a significant increase in FSE immediately after intervention with a small effect size of 0.22 (Table 3; paired t test, p = 0.023). None of the WBV frequency/duration dose conditions was associated with a significant within-condition change in FSE at the immediate, 15-min delayed, or 45-min delayed assessments (Table 3).
Although mean baseline FSE was not different between any of the five sessions, most participants displayed a large degree of variability in baseline spasticity between sessions (Fig. 3). Consequently, to determine whether spasticity severity was associated with responsiveness to WBV, we stratified participants into high spasticity and low spasticity subgroups. Using median baseline FSE of all intervention sessions (46.6°) as the stratification cutoff, participants with baseline FSE < 46.6° were stratified into the high spasticity subgroup, and participants with baseline FSE > 46.6° were stratified into the low spasticity subgroup. There were no significant differences in the change in FSE from baseline to any of the three postintervention assessments (immediate, 15-min delayed, and 45-min delayed) when comparing each WBV frequency/duration dose condition to the sham-control in either the high spasticity (immediate (F = 0.44, p = 0.78), 15-min delayed (F = 0.67, p = 0.61), and 45-min delayed (F = 0.86, p = 0.50)) or low spasticity (immediate (F = 0.92, p = 0.46), 15-min delayed (F = 2.1, p = 0.09), and 45-min delayed (F = 0.34, p = 0.85)) subgroups (Fig. 4).
Baseline first swing excursion (FSE) variability across sessions. Average FSE for each participant in represented by the closed circles while the range of baseline FSE values is denoted by the black lines. The data for participants 14 and 25 represent a single completed session. The data for participants 22 and 33 represent two completed sessions, and the data for participant 30 represent three completed sessions. The data for all other participants represent baseline FSE for all five sessions
Change in mean first swing excursion (FSE) values from baseline to immediate (dark gray bars), 15-min delayed (light gray bars), and 45-min delayed (white bars) post-tests when participants stratified according to baseline spasticity. (A) Participants with high baseline spasticity, FSE < 46.6°. (B) Participants with low baseline spasticity, FSE > 46.6°. Results represent means ± SEM
Within-condition effects were observed for both subgroups. In the high spasticity subgroup, the sham-control and HFSD WBV frequency/duration dose condition were associated with significant within-condition increases in FSE immediately after intervention (Table 4, high spasticity subgroup; paired t test, p = 0.013 and p = 0.048, respectively). Effect sizes for the change in FSE immediately after intervention were small for the HFSD, LFSD, HFLD, and LFLD WBV frequency/duration dose conditions and moderate for the sham-control (Table 4, high spasticity subgroup). At the 15-min delayed assessment, both the HFSD and HFLD WBV frequency/duration dose conditions were associated with significant within-condition increases in FSE (Table 4, high spasticity subgroup; paired t test, p = 0.027 and p = 0.014, respectively). Effect sizes for the change in FSE 15 min after intervention were small for the sham-control and LFSD and LFLD WBV frequency/duration dose conditions, moderate for the HFSD WBV frequency/duration dose condition, and large for the HFLD WBV frequency/duration dose condition (Table 4, high spasticity subgroup). No significant changes in spasticity were observed in the high spasticity subgroup at the 45-min delayed assessment. While effect sizes for the change in FSE 45 min after intervention were small for the sham-control and HFSD and LFLD WBV frequency/duration dose conditions, the effect size was moderate for the HFLD WBV frequency/duration dose condition (Table 4, high spasticity subgroup).
Analysis of within-condition effects for the low spasticity subgroup displayed no significant changes in spasticity immediately after intervention. A small negative effect was observed with the HFSD WBV frequency/duration dose condition immediately after intervention (Table 4, low spasticity subgroup). At the 15-min delayed assessment, the HFSD and HFLD WBV frequency/duration dose conditions were associated with a significant decrease in FSE (Table 4, low spasticity subgroup; paired t test, p = 0.044 and p = 0.021, respectively). A small negative effect was observed with the HFSD and HFLD WBV frequency/duration dose conditions 15 min after intervention. At the 45-min delayed assessment, the HFSD and HFLD WBV frequency/duration dose conditions were associated with a significant decrease in FSE (Table 4, low spasticity subgroup; paired t test, p = 0.020 and p = 0.010, respectively). A small negative effect was observed for all five interventions 45 min after intervention.
Effects of WBV on Walking Speed
Mean baseline walking speed was not different among any of the five intervention sessions (Fig. 5A; one-way ANOVA, F = 0.06, p = 0.992). To determine the effect of each WBV frequency/duration dose condition on walking function, we first compared mean walking speed for each of the four WBV frequency/duration dose conditions (HFSD, LFSD, HFLD, LFLD) to the sham-control. The change in walking speed from baseline to either of the two postintervention assessment time points (immediate and 45-min delayed) was not different when comparing each WBV frequency/duration dose condition to the sham-control at each of the time points (immediate F = 0.34, p = 0.85 and 45-min delayed, F = 0.76, p = 0.55) (Fig. 5A). In regard to within-condition effects, none of the five conditions was associated with a significant within-condition change in walking speed, and effect sizes for each condition were negligible (Table 5). It should be noted that the immediate walking assessment was only completed by a subset of participants (participants 12–35) because this walking assessment time point was added to the study protocol after the first 11 participants completed the study. In addition, some participants found it necessary to empty their bladders immediately after intervention, thus precluding them from the immediate walk. Two participants did not complete any walking assessments because they were unable to complete the 10-m walk without assistance despite meeting all inclusion criteria. The presented walking data (Fig. 5 and Tables 5 and 6) account for the walks that were not completed.
Change in mean walking speed from baseline to immediate (dark gray bars) and 45-min delayed (white bars) post-tests. (A) All participants. (B) Participants with high baseline spasticity, FSE < 46.6°. (C) Participants with low baseline spasticity, FSE > 46.6°. The immediate walk was only completed in a subset of participants as described in the text. Results represent means ± SEM
When participants were stratified according to baseline spasticity (as described in the preceding section), there were no significant differences in the change in walking speed from baseline to either of the two postintervention assessments (immediate and 45-min delayed) when comparing each WBV frequency/duration dose condition to the sham-control in either the high spasticity (immediate F = 0.11, p = 0.98 and 45-min delayed, F = 1.32, p = 0.27) or low spasticity subgroups (immediate F = 0.89, p = 0.48 and 45-min delayed, F = 1.00, p = 0.41) (Fig. 5B and C). For within-condition effects, none of the five conditions was associated with a significant within-condition change in walking speed in either the high spasticity or low spasticity subgroups (Table 6). Within-condition effect sizes were also negligible in both subgroups (Table 6).
Discussion
In the current study, we compared the single-session effects of four different WBV frequency/duration dose conditions to a sham-control intervention to assess their influence on spasticity and walking speed in participants with chronic SCI. While the change in FSE from baseline to the postintervention assessments (immediate, 15-min delayed, and 45-min delayed) was not different from the sham-control condition for any of the WBV doses, a within-condition decrease in spasticity was observed immediately after the sham-control intervention.
When participants were stratified into high and low spasticity subgroups to determine whether baseline spasticity influenced responsiveness, more prominent changes in FSE were observed. For participants with high spasticity, we found that the sham-control and HFSD WBV frequency/duration dose conditions were associated with a significant decrease in spasticity immediately after intervention. The HFSD and HFLD WBV frequency/duration dose conditions were associated with more persistent effects at 15 min after intervention. Moreover, the change in FSE during HFLD WBV was associated with a large effect size for the 15 min after intervention assessment and a moderate effect for the 45 min after intervention. For participants with low spasticity, the HFSD and HFLD frequency/duration dose conditions were associated with a significant increase in spasticity at both delayed postintervention assessments (15min, 45min). No changes in walking speed were observed with any of the five conditions in either the whole group or high and low spasticity subgroups. Based on these results, we conclude that the HFLD WBV frequency/duration dose condition was associated with the largest effect sizes among the five conditions tested.
Frequency-Related Effects
Prior studies of individuals with spasticity have hinted at mechanisms that may underlie the influence of WBV on spasticity. Tendon vibration is associated with a reduction in involuntary muscle contractions, an effect which is attributed to long-lasting presynaptic inhibitory effects on spinal Ia circuits [27]. These presynaptic inhibitory effects are elicited with tendon vibration frequencies of 40 Hz and higher [28], with 80–100 Hz frequencies being common in electrophysiological studies of presynaptic inhibition. In persons with SCI, tendon vibration increases presynaptic inhibition, albeit to a lesser degree than that of nondisabled individuals [12]. Other studies in persons with SCI have suggested that inhibitory effects of vibration may be due to suppression of excitability in flexor reflex afferents [27] or enhancement of reciprocal inhibition [29].
Prior studies in nondisabled participants have demonstrated that WBV activates muscle reflex activity in much the same manner as tendon vibration, and the amplitude of reflex-evoked muscle activity increases with increased WBV frequency [30]. The vibration paradox is a known physiologic effect wherein the tendon vibration evokes a reflex muscle contraction via Ia stretch receptor activation, while concurrently activating inhibitory mechanisms that reduce the size of the reflex contraction [31].There is evidence to suggest that inhibitory effects of WBV arise not from presynaptic inhibition, but rather through homosynaptic postactivation depression [32]. When stimulation is used to activate H-reflexes repeatedly, homosynaptic postsynaptic depression of the H-reflex is observed wherein the magnitude of depression is rate (i.e., frequency) dependent, a phenomenon referred to as low-frequency depression [33]. It seems feasible that the effects of WBV could similarly activate inhibitory mechanisms in a frequency-dependent manner, and this may account for the observation that the higher frequency of WBV was associated with the largest decrease in spasticity.
Sham Effects
We observed that the sham-control condition was associated with a significant reduction in spasticity immediately after intervention for both the whole group and high spasticity subgroup. These results are consistent with published studies indicating that activity and training are associated with a reduction in spasticity [34,35,36]. Indeed, experimental evidence indicates that movement plays an important role in regulating the excitability of spinal cord circuitry as muscle inactivity induced by joint immobilization increases spinal reflex excitability in noninjured individuals [37]. Because sensory afferent activation contributes to the modulation of spinal reflex excitability [1], it is possible that the activity associated with repeated sitting and standing during the sham-control condition mediated the reduction in spasticity observed with our participants. Therefore, reengaging afferent activation that has been lost after SCI due to inactivity may play a role in spasticity reduction. It is important to emphasize, however, that the effects of the sham-control intervention did not persist beyond the immediate postintervention assessment, suggesting that after neurological injury more robust afferent input may be required to reduce spasticity.
Individual Variability
Individuals with SCI indicate that their spasticity varies throughout the day [3]. Likewise, our data indicate that many of our participants experienced large day-to-day variations in spasticity as indicated by the wide range of baseline FSE values between sessions. Due to this variability, we stratified participants into high spasticity and low spasticity subgroups for each condition based on baseline FSE values. In the low spasticity subgroup, the HFSD and HFLD WBV frequency/duration dose conditions were associated with a significant increase in spasticity at both delayed time points, whereas the same two WBV frequency/duration dose conditions were associated with a significant reduction in spasticity for participants with high baseline spasticity at the immediate (HFSD) and 15-min delayed time points (HFSD, HFLD). Taken together, these results suggest that the effects of WBV on stretch-induced spasticity are dependent upon the amount of spasticity that an individual is experiencing. Therefore, selection of a single WBV frequency/duration dose condition may not be the best approach for managing an individual’s spasticity. Instead, the more appropriate strategy may be to select the WBV frequency/duration dose condition based on the amount of spasticity an individual is experiencing at a given time.
Clinical Implications
Research indicates that activity, exercise, and training are associated with modulatory effects that reduce spasticity in persons with spinal cord injury [35]. Conversely, a study in nondisabled individuals suggests the lack of activity is associated with a period of immobilization results in loss of reflex modulation, and the development of hyperreflexic responses that parallel those observed in individuals with neurologic conditions (37). For individuals with mobility limitations, it seems possible that electrical stimulation or vibration may represent a viable approach to mimicking the effects of activity to elicit neuromodulation [7, 8, 38, 39]. The value of these interventions when used in combination with training warrants additional study.
Limitations
The current study evaluated only stretch-induced spasticity in a single muscle group, the quadriceps. While the pendulum test has been validated as a robust and repeatable measure of velocity-dependent stretch reflex excitability, spasticity manifests in many ways beyond the classical velocity-dependent presentation evaluated in this study. Recent survey research indicates that persons with SCI report stiffness as the most problematic aspect of their spasticity among the common characteristics of hyperreflexia, spasms, and stiffness [3]. While our data suggest that WBV reduces stretch reflex excitability in individuals with more severe spasticity, the effects of WBV on other aspects of spasticity, including stiffness, remain unknown. Additional studies are warranted to provide a more comprehensive understanding of the effects of WBV on spasticity in persons with SCI.
Because the goal of this study was to evaluate the comparative effects of multiple WBV frequency/duration dose conditions, we tested only a single session of each intervention. This single-session intervention application may account for the lack of change in walking speed in the current study, as 12 sessions of WBV have been previously shown to improve walking function in persons with SCI [19]. Prior work from our lab suggests that a multi-week regimen of WBV leads to a progressive reduction in spasticity [7]. Consequently, we also predict that HFLD WBV will lead to cumulative effects in our planned multi-session study of WBV.
Conclusions
Individuals with SCI often experience spasticity that can negatively affect their function and quality of life. Although anti-spasmodic medications are commonly used to treat spasticity, they do not always effectively manage an individual’s spasticity and are often accompanied by negative side effects. Consequently, the use of physical therapeutic interventions, including WBV, as an alternative treatment for spasticity is a growing area of interest in rehabilitation research. Our data suggest that, of the frequency/duration dose conditions tested, higher frequency (50 Hz) WBV applied for longer durations (6 min) was most effective for reduction of stretch-induced quadriceps spasticity in persons with SCI. However, it should be noted that those with high spasticity are likely to have a greater reduction in tone than those with low spasticity. Future studies should be dedicated to identifying the cumulative effects of WBV, perhaps in combination with other interventions, on characteristics of spasticity beyond velocity-dependent stretch reflexes.
References
Trompetto C, Marinelli L, Mori L, Pelosin E, Curra A, Molfetta L, et al. Pathophysiology of spasticity: implications for neurorehabilitation. Biomed Res Int. 2014;2014:354906.
Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and Effect of Problematic Spasticity After Traumatic Spinal Cord Injury. Archives of physical medicine and rehabilitation. 2017;98(6):1132–8.
McKay WB, Sweatman WM, Field-Fote EC. The experience of spasticity after spinal cord injury: perceived characteristics and impact on daily life. Spinal Cord. 2018.
Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30(12):1303–13.
Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27(1–2):2–6.
Theriault ER, Huang V, Whiteneck G, Dijkers MP, Harel NY. Antispasmodic medications may be associated with reduced recovery during inpatient rehabilitation after traumatic spinal cord injury. The journal of spinal cord medicine. 2018;41(1):63–71.
Ness LL, Field-Fote EC. Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor Neurol Neurosci. 2009;27(6):621–31.
Estes SP, Iddings JA, Field-Fote EC. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Front Neurol. 2017;8:17.
Khan F, Amatya B, Bensmail D, Yelnik A. Non-pharmacological interventions for spasticity in adults: An overview of systematic reviews. Ann Phys Rehabil Med. 2017.
Burke D, Ashby P. Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? J Neurol Sci. 1972;15(3):321–6.
Ashby P, Verrier M, Lightfoot E. Segmental reflex pathways in spinal shock and spinal spasticity in man. J Neurol Neurosurg Psychiatry. 1974;37(12):1352–60.
Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalography and clinical neurophysiology. 1993;89(3):177–86.
Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods. 1997;74(2):189–99.
Pang MY, Lau RW, Yip SP. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: a randomized controlled trial. Eur J Phys Rehabil Med. 2013;49(4):439–50.
Sadeghi M, Sawatzky B. Effects of vibration on spasticity in individuals with spinal cord injury: a scoping systematic review. Am J Phys Med Rehabil. 2014;93(11):995–1007.
Field-Fote E, Ness LL, Ionno M. Vibration elicits involuntary, step-like behavior in individuals with spinal cord injury. Neurorehabilitation and neural repair. 2012;26(7):861–9.
Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movements evoked by leg muscle vibration in humans. The European journal of neuroscience. 1998;10(5):1608–12.
Ivanenko YP, Grasso R, Lacquaniti F. Influence of leg muscle vibration on human walking. Journal of neurophysiology. 2000;84(4):1737–47.
Ness LL, Field-Fote EC. Whole-body vibration improves walking function in individuals with spinal cord injury: a pilot study. Gait Posture. 2009;30(4):436–40.
Choi W, Han D, Kim J, Lee S. Whole-Body Vibration Combined with Treadmill Training Improves Walking Performance in Post-Stroke Patients: A Randomized Controlled Trial. Med Sci Monit. 2017;23:4918–25.
van Nes IJ, Geurts AC, Hendricks HT, Duysens J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: preliminary evidence. Am J Phys Med Rehabil. 2004;83(11):867–73.
Nance PW, Bugaresti J, Shellenberger K, Sheremata W, Martinez-Arizala A. Efficacy and safety of tizanidine in the treatment of spasticity in patients with spinal cord injury. North American Tizanidine Study Group. Neurology. 1994;44(11 Suppl 9):S44–51; discussion S-2.
Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. The journal of spinal cord medicine. 2014;37(2):202–11.
de Azevedo ER, Maria RM, Alonso KC, Cliquet A, Jr. Posture Influence on the Pendulum Test of Spasticity in Patients with Spinal Cord Injury. Artif Organs. 2015;39(12):1033–7.
Fowler EG, Nwigwe AI, Ho TW. Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Developmental medicine and child neurology. 2000;42(3):182–9.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press. 1969.
Butler JE, Godfrey S, Thomas CK. Depression of involuntary activity in muscles paralyzed by spinal cord injury. Muscle Nerve. 2006;33(5):637–44.
Ashby P, Stalberg E, Winkler T, Hunter JP. Further observations on the depression of group Ia facilitation of motoneurons by vibration in man. Experimental brain research. 1987;69(1):1–6.
Perez MA, Floeter MK, Field-Fote EC. Repetitive Sensory Input Increases Reciprocal Ia Inhibition In Individuals With Incomplete Spinal Cord Injury. Journal of Neurologic Physical Therapy. 2004;28:114–21.
Cidem M, Karacan I, Cakar HI, Cidem M, Sebik O, Yilmaz G, et al. Vibration parameters affecting vibration-induced reflex muscle activity. Somatosens Mot Res. 2017;34(1):47–51.
Desmedt JE. Mechanisms of vibration-induced inhibition or potentiation: tonic vibration reflex and vibration paradox in man. Adv Neurol. 1983;39:671–83.
Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol (1985). 2012;112(3):388–95.
Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, et al. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123 ( Pt 8):1688–702.
Phadke CP, Flynn SM, Thompson FJ, Behrman AL, Trimble MH, Kukulka CG. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Archives of physical medicine and rehabilitation. 2009;90(7):1218–28.
Manella KJ, Field-Fote EC. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci. 2013;31(5):633–46.
Knikou M, Mummidisetty CK. Locomotor training improves premotoneuronal control after chronic spinal cord injury. Journal of neurophysiology. 2014;111(11):2264–75.
Lundbye-Jensen J, Nielsen JB. Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol. 2008;586(17):4121–35.
Shields RK, Dudley-Javoroski S, Oza PD. Low-frequency H-reflex depression in trained human soleus after spinal cord injury. Neuroscience letters. 2011;499(2):88–92.
Sivaramakrishnan A, Solomon JM, Manikandan N. Comparison of transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES) for spasticity in spinal cord injury—A pilot randomized cross-over trial. The journal of spinal cord medicine. 2017:1–15.
Acknowledgments
The authors would like to express their gratitude to the research participants who volunteered their time to participate in this study. They would also like to thank Temple Moore, OTR/L, Elizabeth Sasso-Lance, PT, DPT, and Rachel Betzler for their recruitment efforts along with Nicholas Evans, MHS, CEP, and Adam Holzwarth, PT, DPT for their assistance with conducting interventions,This study was supported by the NIH National Institute of Child Health and Human Development (NICHD) R01 HD079009-02 (EF-F).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 1225 kb)
Rights and permissions
About this article
Cite this article
Estes, S., Iddings, J.A., Ray, S. et al. Comparison of Single-Session Dose Response Effects of Whole Body Vibration on Spasticity and Walking Speed in Persons with Spinal Cord Injury. Neurotherapeutics 15, 684–696 (2018). https://doi.org/10.1007/s13311-018-0644-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-018-0644-1