Abstract
Though seemingly distinct and autonomous, emerging evidence suggests there is a bidirectional interaction between the intestinal microbiota and the brain. This crosstalk may play a substantial role in neurologic diseases, including anxiety, depression, autism, multiple sclerosis, Parkinson’s disease, and, potentially, Alzheimer’s disease. Long hypothesized by Metchnikoff and others well over 100 years ago, investigations into the mind–microbe axis is now seeing a rapid resurgence of research. If specific pathways and mechanisms of interaction are understood, it could have broad therapeutic potential, as the microbiome is environmentally acquired and can be modified to promote health. This review will discuss immune, endocrine, and neural system pathways that interconnect the gut microbiota to central nervous system and discuss how these findings might be applied to neurologic disease.
Similar content being viewed by others
Intestinal Microbiota Location and Functional Capabilities
The intestinal microbiota refers to the total bacteria, viruses, fungi, and microscopic protozoa that inhabit the gastrointestinal (GI) tract. In humans, it is estimated to be composed of > 10 trillion cells [1], with 100s to 1000s of microbial species per individual, and the collective genes within the microbiome outnumber genes in the human genome 100:1 [2]. This vast genetic diversity encodes a myriad of functions that confer a benefit to their host that have been selected for over our entire evolutionary history.
The intestinal microbiota provide many supportive functions and can regulate host physiology. For example, the microbiota enhance energy extraction from the diet, produce vitamins, educate the immune system, protect from infectious agents, maintain gut barrier integrity, and guide metabolic and neurologic development [3,4,5,6,7]. The greatest numbers of microbiota inhabit the large intestine, with up to 1012 cells per milliliter of intestinal contents [7]. The enterohepatic circulation between the GI tract and the liver provides direct access of host metabolites to the microbiota, and vice versa. Microbial secreted products, including neurotransmitters and immunomodulators, can enter circulation and act on distant organ systems [8]. In addition, the microbiota can chemically transform host-derived molecules and influence endocrine and metabolic function by regulating the excretion or reuptake of hormones and cholesterol [9, 10]. Finally, the intestinal microbiota also plays a role in therapeutic drug activity, by either activating or inactivating these exogenous compounds [11].
Humans are initially colonized by microbiota at birth, which undergo a series of ecological progression during infancy and early childhood until it establishes an adult-like composition by 3 years of age [12]. There is conflicting evidence of whether microbial colonization begins in utero [13, 14] or at birth; however, it is plausible that exposure to microbial products entering the bloodstream may affect the developing fetus [15]. Several factors influence the acquisition and maturation of the microbiota, including birth mode (vaginal delivery or cesarian section), antibiotics, breastfeeding, diet, and environmental exposure to microbes [16,17,18]. Alterations in early-life microbiota colonization can have lasting effects on metabolism [19], immunity [20, 21], and neurologic function [22, 23].
The adult microbiota in a healthy adult is relatively stable and specific to an individual, and the composition is governed by both the environment and genetics [24]. Host genes that encode functions related to immunity, metabolism, gut motility, and bacterial adhesion can shape lifelong microbiota colonization [25]. Despite the role that genetics play, identical twins can have different microbiota that can contribute to chronic diseases [26]. Environmental factors such as diet, exercise, maternal environment, mode of delivery, and exposure to sources of new microbiota (hygiene or probiotics) play a role in modulating and maintaining the microbiota composition [27]. As behavioral patterns and routines normalize in early adulthood, the microbiota composition becomes more stable until the emergence of immunosenescence in advanced aging [24]. While a person usually maintains a relatively consistent microbiota over time, there still is day-to-day variation within an individual [28], and dramatic changes can occur following large disruptions [29]. Common sources of disruption include antibiotics, infection, or colonization by foreign commensal microbes, large changes in diet [30], or noninfectious disease that alters the GI function. Depending on the magnitude and length of disruption, the microbiota is capable of recovering and returning to its original composition once the selective pressure is released; however, repeated disturbances can impair recovery and this may have a downstream effect on host physiology [31,32,33].
Several principles from clinical infectious disease can be instructive for understanding complex microbiota–host interactions. First, children and the elderly are at a higher risk of infection due to either an immature or weakened immune system. Similarly, these ages represent time periods of decreased microbiota stability and enhanced vulnerability to microbiota disruption [18, 24, 34]. Intriguingly, there are also specific windows of vulnerability for the presentation of various neurologic diseases throughout the lifespan [22]. Whether or not this timing is related to changes within the microbiota is still to be determined.
Lines of Microbiota–Gut–Brain Communication
The intestinal microbiota and the central nervous system (CNS) are connected via multiple bidirectional pathways involving neural, endocrine, and immune signaling (Fig. 1).
Bidirectional lines of communication between the gut microbiota and the brain. Neural, endocrine, and immune pathways mediate signals between the central nervous system (CNS) and the intestinal microbiota. The microbiota can produce a variety of neuro- and immunomodulatory substances that can either act locally on immune populations and enteroendocrine cells in the gut, or modulate distant functions in the CNS. This can alter mood, behavior, and neuroinflammatory responses. Stress signals from the brain are conducted via the efferent nerves, as well as the hypothalamic–pituitary–adrenal (HPA) axis, which can alter gastrointestinal function and microbiota composition. PYY = peptide YY; 5-HT, 5-hydroxytryptamine; SCFA = short-chain fatty acid; LPS = lipopolysaccharide
The microbiota can induce cells in the GI tract to produce neurotransmitters or digestive hormones that alter the brain and behavior [8, 35,36,37]. They can also secrete neuroactive metabolites into the circulation, modulate local immune populations that traffic to the CNS, and stimulate the vagus nerve to have an effect on behavior [38]. In turn, the CNS can control the gut microbiota via adrenergic nerve signaling, primarily affecting intestinal motility and by the influence of neurotransmitters on the immune mediators that shape microbiota composition and function [8].
Microbiota Influence on Neural Signaling
Afferent and Efferent Neural Signaling
The GI tract is highly enervated and is one of the major signaling pathways connecting the microbiota to the brain. The enteric nervous system, within the autonomic nervous system, contains both afferent (from the GI tract to the CNS) and efferent (from the CNS to the GI tract) functions [39]. Efferent neural signals are conducted from the CNS to the GI tract and can modulate GI motility, secretion, and epithelial permeability, which modify the physical environment that the microbiota inhabit, thus affecting its composition. The vagus afferent nerves transmit signals from the GI tract to the CNS and are capable of recognizing microbial products or cell wall components.
Probiotic bacteria have been shown to alter stress responses and behavior in a manner dependent on the vagus nerve. Bravo et al. [40] demonstrated that Lactobacillus rhamnosus could reduce anxiety and depressive-like behavior, as evidenced by spending more time in the open arm of the elevated plus maze, and had less immobility during the forced swim test. Behavioral changes were accompanied by altered levels of gamma-aminobutyric acid mRNA gene expression, which varied by brain region, and was dependent on signaling via the vagus nerve as all effects were lost in vagotomized mice treated with L. rhamnosus [40]. Pain and resulting anxiolytic responses can be conducted via the vagal nerve. Administering Bifidobacterium longum to mice reduced anxiety in mice given dextran sodium sulfate colitis, but the effect was lost when the vagus nerve was severed [41]. Probiotics can also communicate with other components of the enteric nervous system. Lactobacillus reuteri targeted ion channels in enteric sensory neurons leading to increased action potential and excitability, which may have downstream effects on motility and pain perception [42].
Neurotransmitters
Members of the gastrointestinal microbiota are capable of secreting neuroactive peptides, including gamma-aminobutyric acid, noradrenaline, dopamine, acetylcholine, and 5-hydroxytryptamine [8]. However, whether these microbial metabolites are produced at an appreciable level compared with host production or whether they act locally at the gut level or can be transported systemically across the blood–brain barrier is unknown [39]. Over 90% of the serotonin (5-hydroxytryptamine) in the body is synthesized in the GI tract, primarily by the enterochromaffin (EC) cells, a subtype of enteroendocrine cells, which regulate GI motility, secretion, nausea, and visceral hypersensitivity [43]. In order to dissect cell-specific responses, Bellono et al. [43] generated intestinal organoids with EC cells and demonstrated that microbial products, primarily isovalerate and to a lesser extent butyrate, signaled through the olfactory receptor 558. Furthermore, they showed that EC cells are electrically excitable and are in direct proximity to serotonin-sensitive afferent nerve fibers; thus, EC cells in the intestinal epithelium have a major transduction role in detecting microbial products [43]. Isovalerate is a short-chain fatty acid (SCFA) usually produced in low amounts compared with acetate, propionate, and butyrate, and isovalerate accumulation is associated with GI pain and postinfectious irritable bowel syndrome [44,45,46]. Thus, the EC cells may serve as rapid surveillance for imbalances within the microbiota that lead to the enrichment of this toxic metabolite.
Neurotoxins
Bacteria are capable of producing potent neurotoxins, as seen with botulism and tetanus, which can result from an intoxication caused by contaminated food or from a soft tissue infection, rather than stable colonization at an anatomic site with resident microbiota. A rare but serious neurologic complication from early-life microbiota colonization is infant botulism, in which Clostridium botulinum, Clostridium butyricum, or Clostridium baratii colonize the GI tract and secrete the botulinum toxin at low levels, initially presenting as hypotonia and reduced feeding, and leading to paralysis, respiratory distress, and, in some cases, death [47, 48]. The immature microbiota of an infant is more vulnerable to invasion of these pathogens, whereas this is rarely observed in adults, likely owing to the pathogen resistance conferred from a developed microbiota. While infant botulism is one example of toxin produced in the intestine affecting the CNS, it is plausible that additional species within the microbiota can secrete highly potent neuroactive chemicals that have not yet been identified.
Microbiota Regulation of Endocrine Signaling
Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis is a cascade of hormonal signaling in response to stress and can be shaped by the microbiota during critical developmental periods. The hypothalamus secretes corticotropin-releasing factor, which stimulates the pituitary gland to secrete adrenocorticotropic hormone, which then induces the adrenal cortex of the kidneys to secrete glucocorticoids, including cortisol and corticosterone, the main glucocorticoid in humans and mice, respectively. Germ-free (GF) mice show elevated HPA responses when challenged with a restraint stress, including elevated hypothalamic corticotropin-releasing factor gene expression and protein levels, decreased cortical and hippocampal brain-derived neurotrophic factor, and elevated plasma adrenocorticotropic hormone and corticosterone [49]. Changes in the HPA axis were both developmentally and microbe dependent. Adolescent GF mice colonized with specific-pathogen-free microbiota at 6 weeks of age restored HPA signaling, whereas adult GF mice colonized at 8 or 14 weeks of age continued to show exaggerated stress responses. In addition, monocolonization with the infant commensal bacteria Bifidobacterium infantis lowered stress responses compared with GF mice, whereas an invasive strain of enteropathogenic Escherichia coli increased stress responses and proinflammatory interleukin (IL)-1β and IL-6 cytokine secretion. This was critically mediated by the invasive characteristics of enteropathogenic Escherichia coli as evidenced by the fact that a mutant strain lacking the translocated intimin receptor gene that confers invasive properties did not elevate stress responses [49]. These studies provided evidence that individual strains of bacteria within the microbiota can either positively or negatively regulate the HPA axis and the microbiota as a whole participates in developmentally programming stress responses.
Glucocorticoids and the HPA axis also play a role in immunomodulation, which enhances survival from infectious diseases [50]. Depletion of HPA signaling via chemical inhibitors or adrenalectomy decreased survival in mice challenged with sublethal doses of microbial toxins, including lipopolysaccharide (LPS), shiga toxin, and Staphylococcus aureus superantignen enterotoxin B [50]. Conversely, overexpression of glucocorticoid receptor (GR) protected mice from endotoxic shock when challenged with LPS [51]. As a little-explored mechanism of virulence, microbial toxins can disrupt GR signaling. Secreted toxins from Shigella (shiga toxin), Bacillus anthracis (anthrax lethal toxin), Clostridium difficile (TcdA and TcdB), Clostridium sordellii (TscL), and S. aureus (superantigens, toxic shock syndrome toxin 1, and enterotoxin E), as well as cell wall components (endotoxin, LPS), have been shown to repress GR-mediated gene activation [50]. Whether subclinical toxin production is a mechanism by which resident microbiota regulate stress levels has not been explored.
Peptide YY
Digestive hormones that control appetite and GI motility are also important bidirectional mediators of microbiota–brain endocrine signaling [52]. The satiety-inducing hormone peptide YY (PYY) is synthesized primarily in the GI tract and is transported to the brain. It is produced by enteroendocrine cells in response to the G protein-coupled receptor Ffar3 (Gpr41)-sensing dietary proteins and fats and microbially derived SCFAs [53]. Thus, the microbiota metabolic end products can directly modulate PYY levels. PYY affects the brain, feeding behavior, and the GI tract by triggering satiety, reducing food intake, and slowing GI motility, factors that may affect the microbiota. Altering the microbial composition with low-dose penicillin has been shown to decrease PYY levels, which is associated with increased food intake and microbe-induced obesity [19].
Microbiota–Immune Signaling Effects on the CNS
Immunologic Development
The intestinal microbiota plays a key role in immune development and function [6, 54], and specific microbes have different mechanisms of action to influence particular immune cell subsets [55]. For example, a Gram-positive spore-forming anaerobic bacteria referred to as segmented filamentous bacteria can adhere to enterocytes in the distal small intestine (ileum) and this direct contact induces the differentiation of T helper (Th)17 cells [56]. Th17 cells are an important cell type involved not only in protection from intestinal infection, but also in the pathogenesis of proinflammatory diseases, such as multiple sclerosis (MS) and rheumatoid arthritis [57]. Conversely, closely related spore-forming anaerobic bacteria within Clostridial clusters IV and XIV produce abundant butyrate from the fermentation of carbohydrates, which can induce the proliferation of T-regulatory (Treg) cells [58]. Bacteroides fragilis, a Gram-negative anaerobic bacteria, can also induce the proliferation of Treg cells by a mechanism linked to the cell wall component polysaccharide A [59], rather than a secreted product, demonstrating that diverse bacteria can act by varied mechanisms to produce a similar immunologic response.
In addition to differences between species there can also be critical strain-dependent immunomodulation that effects disease outcome. For example, Helicobacter pylori strains vary in their ability to cause peptic ulcer disease and gastric adenocarcinoma based on the presence of a pathogenicity island containing a type IV secretion system that injects the CagA product into epithelial cells, inducing inflammation and altering a number of host signaling pathways [60, 61]. Escherichia coli strains can be either beneficial commensal organisms or cause serious infectious diseases, depending on whether they have acquired mobile genetic elements from Shigella or other pathogenic bacteria [62, 63]. Finally, several species of Clostridium, including C. tetani (agent of tetanus) and C. botulinum (causative agent of botulism), can encode different types of neurotoxins and exhibit considerable strain diversity [64], which determine host range (e.g., humans, birds, etc.) and recovery following treatment [65].
Metabolic Mediators of Neuroimmune Function
SCFAs
The GI microbiota produce large quantities of SCFAs, reaching up to 50 to 200 mM in the large intestine [66]. The 3 major SCFAs produced are acetate, propionate, and butyrate, which accumulate in an approximate ratio of 60:20:20 and have differing metabolic and signaling fates [67]. The quantity of SCFAs depends on the composition of the microbiota and the amount of complex carbohydrates in the diet. Simple carbohydrates, such as glucose, fructose, and starch, are primarily absorbed in the small intestine, and thus contribute little to the carbohydrate fuel source for the microbiota. Complex carbohydrates escape host absorption and reach the large intestine where they are first broken down to monomeric sugars, then fermented to SCFA [68]. Minor amounts of short-chain, as well as branched-chain, fatty acids can be produced from microbial breakdown of protein when carbohydrate levels in the large intestine are low. Acetate is made by a many of diverse types of microbes, including Bacteroides, Clostridium, Akkermansia, and Bifidobacterium [68], whereas butyrate is made by fewer types of microbes, mostly within Clostridial cluster IV (family Ruminococcaceae, including the genera Faecalibacterium, Anaerotruncus, and Subdoligranulum) and Clostridial cluster XIV (family Lachnospiraceae, including the genera Roseburia, Anaerostipes, Coprococcus, and some species of Eubacterium) [69]. Propionate is produced from both Gram-negative bacteria, including Bacteroides, Veillonella, Dialister, and Salmonella, and Gram-positive bacteria, including Coprococcus, Roseburia, and Ruminococcus [68].
Emerging evidence indicates that SCFAs play a role in neuroimmune homeostasis, as well as neurodegenerative diseases [70]; however, first it is informative to consider the general physiological fates of microbial SCFA in the host. Acetate can contribute to cholesterol synthesis in the liver and accumulates in the bloodstream as it is the predominate SCFA produced. Butyrate is the major fuel source of the colonocytes, preferred over glucose or glutamine. Colonocytes can utilize 70% to 90% of the butyrate pool and thus little of this SFCA enters circulation. Butyrate also acts as a histone deacetylase inhibitor, which confers protection against colon cancer [67] and suppresses proinflammatory macrophages in the lamina propria [71]. Propionate is utilized for hepatic gluconeogenesis and inhibits cholesterol synthesis. The 3 predominant SCFAs also signal through G protein-coupled receptors (GPR), known as free fatty acid receptors (FFAR)2 and FFAR3 (previously named GPR43 and GPR41, respectively), as well as GPR109. FFAR2 primarily responds to acetate and propionate, and promotes the expansion and differentiation of Treg cells and neutrophil chemotaxis [68]. FFAR3 has the highest affinity for propionate, next for butyrate, and only responds weakly to acetate, and can promote the expansion of dendritic and T-cell precursors from the bone marrow [68]. GPR109A responds to butyrate and promotes the generation of IL-10 secreting Treg cells [72].
SCFA and Microglia
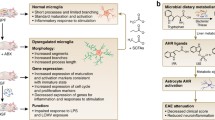
SCFAs have recently been shown to influence the CNS by modulating microglia during maturation, homeostasis, and disease [70, 73]. Microglia are 1 of the 4 predominant cell types in the brain (neuron, glia, microglia, astrocyte), and are the principal immune cell of the brain, serving a macrophage-like function to defend against invading pathogens and scavenge debris [74]. Erny et al. [70] demonstrated that GF mice exhibit altered microglia gene expression, protein production, and morphology, which resemble an immature or dysfunctional state [70]. Microglia isolated from GF mice have decreased gene expression related to pathogen recognition (TRIM family genes), cell activation (Mapk8, Fcgr2b, IL1a, Ly86, CD86, Hif1a, Jak3, Stat1), and maturation (Major histocompatability complex I-related molecule β2 microglobulin), and increased expression in genes that are typically downregulated in adult mice related to transcriptional inhibition (Nfkbib), cell survival (Spi1, and Csf1r), and proliferation (Ddit4 and Iqgap1). At the protein level, surface markers CSF1R, F4/80, and CD31, which also typically decrease during normal maturation, were increased in GF adult mice. Increased numbers of microglia and markers of cell proliferation (Ddit4+ and Iba-1+Ki67+) were observed in GF mice as detected by histopathology. GF mice also showed altered microglia morphology, including longer dendrite processes, and increased branching and cell size. Altogether, these data suggest that the microbiota plays a role in activating microglia while limiting their population and cell size in the brain.
Remarkably, microglial homeostatic changes associated with the absence of microbiota, including gene expression, protein production, and morphologic changes, could be reversed by administering SCFAs via the drinking water [70]. Furthermore, deletion of the SCFA receptor FFAR2 from all cells led to similar changes in microglial morphology as in GF mice, further implicating SCFA as a key mediator of microglia populations. The authors also investigated the effect of microbiota in microglia response to challenge. Despite the more numerous and larger cells, microglia from GF mice had impaired responses to LPS or infection with lymphocytic choriomeningitis virus. Based on this evidence, Erny et al. [70] postulate that the microbiota guide microglia maturation and maintain a homeostatic “never resting” state in which cells are ready to fight invading pathogens or respond to danger signals, and their evidence suggests that SCFA signaling mediates this process.
SCFAs were also important in a in a model of Parkinson’s disease (PD) using α-synuclein overexpressing (ASO) mice. ASO mice that were deficient in microbiota (GF or antibiotic-treated) had fewer motor deficits than their conventional counterparts [73]. Along with improved motor function, GF ASO mice had morphological changes in microglial populations that resembled less-activated microglia (including a decreased cell diameter, with increased and longer processes), as well as lower levels of the proinflammatory cytokines IL-6 and tumor necrosis factor-α in brain regions relevant for PD (caudoputamen and inferior midbrain). The authors tested whether this effect was mediated by immunologic components of microbial cell walls or by microbial secreted products. Transfer of heat-killed bacteria only partially restored the expected motor deficits in the ASO mice, whereas SCFAs fully restored the motor dysfunctions, as well as the morphological changes, in microglia. Whether or not other cell types are linked with this effect is unknown. Nonetheless, the authors clearly demonstrate how microbial products can contribute to disease pathogenesis by influencing the major immune cells in the brain. In order to test whether patients with PD harbored a microbiota that contributes to the disease, they transferred the microbiota of 6 new-onset, treatment-naïve patients with PD and the microbiota of 6 healthy controls to groups of GF mice. In 5 of the 6 pairs, the PD microbiota lead to worse motor deficits versus the healthy control microbiota, providing the first causal evidence that the microbiome may actively contribute to PD disease pathogenesis.
Tryptophan Metabolites
The microbiota also plays an important role in amino-acid metabolism in the gut, which can influence neuroinflammatory diseases. Particular bacteria, including L. reuteri, can break down tryptophan into indole, which is then converted by the host metabolism to indole-3-sulfate, indole-3-aldehyde, and indole-3-propionic acid, which is then transported across the blood–brain barrier to suppress neuroinflammation in astrocytes in the brain and improve outcomes in experimental autoimmune encephalomyelitis (EAE) [75, 76]. Tryptophan is also utilized to synthesize serotonin, and low levels have been linked to clinical depression [77]. GF mice have higher levels of tryptophan and serotonin [78], suggesting that the microbiota amino-acid metabolism can regulate the levels of neuroactive transmitters. Disrupting the microbiota with antibiotics reduced anxiety but increased cognitive defects and was associated with elevated tryptophan and depleted its metabolite, kynurenin [79]. In total, studies are beginning to shed light on microbial tryptophan metabolites as key mediators that can influence both immune and neurological function.
Neurodegenerative Disorders
Immune-Mediated Neurologic Diseases
The microbiota play a critical role in immune-mediated neurologic diseases, including MS and PD, and strong evidence comes from animal models. GF mice resist both induced and spontaneous EAE, the animal model for MS [80, 81], and was associated with reduced pathogenic Th17 cells in the intestinal lamina propria, as well as reduced B-cell recruitment and production of myelin oligodedrocyte glycoprotein autoantibodies in a spontaneous model of EAE [80]. In an inducible model of EAE, the protection in GF mice was linked to a reduction in IL-17 and interferon-γ secretion with a concomitant increase in CD25+FoxP3+ Treg cells, suggesting that the microbiota played an active role in promoting immune responses necessary for disease pathogenesis. Similarly, broad-spectrum antibiotic treatment reduces EAE severity through increasing Treg cells and cytokines IL-10 and IL-13, and decreasing proinflammatory cytokines interferon-γ, tumor necrosis factor-α, IL-6, and IL-17 [82, 83]. Intriguingly, effective control of EAE has only been achieved when high-dose, broad-spectrum antibiotics are applied before the onset of symptoms. In one study of relapsing-remitting EAE, later antibiotic treatment did not reduce the severity of EAE relapses, suggesting that the interaction between the microbiota and the immune system play a key role in disease initiation [84]. Few studies have examined the use of antibiotics in the MS population.
Animal experiments utilize antibiotics for a near elimination of microbiota and differ from antibiotic use to treat infection in the human population. Antibiotic use in adulthood has been linked to an altered risk for developing MS; however, there are conflicting results. One study of 163 MS cases with up to 10 matched controls in the UK found a significant reduction in MS risk in patients receiving > 14 days of penicillin, but no change for other antibiotic classes or shorter exposures [85]. A second study in the Danish population of > 3000 cases with > 30,000 controls found that multiple classes of antibiotics were associated with an elevated risk of MS [86]. A third study of 829 patients with MS and 2441 controls in the US population found no association with antibiotics and the risk of MS, but an association between MS and respiratory tract allergies, and an association between elevated antibiotic use in subjects with respiratory tract allergies, which may be a confounding variable not addressed in other studies [87].
MS
MS is an immune-mediated demyelinating disease that leads to motor dysfunction and cognitive impairment. Recently, we and others have detected alterations in the microbiota of patients with relapsing-remitting MS that are associated with disease status (active vs in remission), disease-modifying treatment, and immunologic changes [88,89,90,91]. In a cohort of approximately 60 patients with relapsing-remitting MS in remission versus > 40 healthy controls, we detected a marked increase in Akkermansia muciniphila and Methanobrevibacter smithii, and a decrease in Butyricimonas, utilizing 2 independent sequencing platforms [88]. Strikingly, these MS-associated changes were reversed in patients on disease-modifying therapies, suggesting that treating MS could also correct changes within the microbiome. Investigating immunologic changes in circulating T cells and monocytes, patients with MS had increased gene expression related to proinflammatory immune pathways, including interferon, Toll-like receptor, IL-6 signaling, and dendritic cell maturation, and both Akkermansia and Methanobrevibacter populations in patients with MS correlated with proinflammatory immune genes; however, this association was lost in healthy controls. This suggests that there may by a direct link with MS-associated microbiota and related changes in systemic immunity. Akkermansia is a known mucin-degrading bacterium that may alter gut barrier function [92]. While it is associated with the promotion of metabolic health in mice [93], it is a relatively recently discovered bacteria and thus would have little prior clinical history [94]. Recently, the first report of Akkermansia bloodstream infection has been reported, first implicating this gut commensal as a potential pathogen [95]. Methanobrevibacter smithii is the major methane producer within the human GI tract that can adhere tightly to the mucosa and is associated with inflammatory diseases, including periodontitis, asthma, and inflammatory bowel disease, which may be due to immunogenic lipid components in its cell wall [96,97,98,99,100]. In addition to detecting changes within the representation of 16S rRNA genes from Methanobrevibacter, we also detected increases in breath methane levels in patients with MS, indicating that there were metabolically active methanogens enriched in MS. Butyricimonas, which was depleted in patients with MS, produces butyrate in the gut, which can induce populations of Treg cells. Decreased butyrate producers belonging to Clostridia IV and XVIa clusters have been detected in another investigation of the MS microbiota [89], suggesting that while exact changes in microbial genera may not be the same, there are consistent changes across microbial function.
Whether or not alterations within the microbiota drive disease pathogenesis in MS or whether the altered immune status results in altered microbiota is an active area of investigation. Recently, Berer et al. [101] evaluated the microbiota in 34 pairs of monozygotic twins that were discordant for MS and detected enrichment in Akkermansia in the twin with MS, consistent with prior studies [88]. The authors further demonstrated that the microbiota could increase disease severity when transferred to GF mice that develop spontaneous CNS autoimmune disease, first suggesting that the altered MS microbiota could directly contribute to impairments in the CNS. This effect was dependent on the healthy twin microbiota inducing protective levels of IL-10, suggesting that the MS microbiota may be lacking critically beneficial microbiota that regulate autoimmunity [101]. In separate cohort, Cekanaviciute et al. [102] also demonstrated that microbiota from untreated patients with MS had higher levels of Akkermansia and GF mice colonized with MS-associated microbiota led to worse disease severity in an inducible model of EAE. This study found that changes within the microbiota, including increased Akkermansia and Acinetobacter, were associated with increasing proinflammatory responses in human peripheral blood mononuclear cells and in monocolonized mice. Conversely, Parabacteroides, which was reduced in patients with MS, induced regulatory cells in mice.
PD
PD is a neurodegenerative disorder characterized by motor impairments, as well as nonmotor symptoms that include constipation, anxiety, depression, and sleep behavior disorder [103]. Disease pathogenesis includes loss of striatal dopamine and is thought to be mediated by aggregation of α-synuclein at the presynaptic terminals of neurons, which may impair vesicular release [104]. Nearly 50% of patients with PD exhibit symptoms of constipation, which often precedes the diagnosis of PD and onset of motor deficits [105], and constipation is a risk factor for later development of the disease. Patients with PD have also been found to have reduced intestinal barrier function, which may increase their exposure to microbial products [104]. Furthermore, individuals that later go on to develop PD show elevated levels of α-synuclein in their GI tract before disease onset, suggesting that this may be a focal point of disease initiation; however, this topic is still under debate [106]. Studies have detected changes in the microbiota of patients with PD, as well as microbial function and metabolites. Patients with PD have been reported to have low Prevotella populations, as well a SCFA-producing microbiota of Faecalibacterium prausnitzii and Clostridial IV cluster [107,108,109,110], and have lowered SCFA metabolites present in the gut than healthy controls [107]. At a functional level, PD microbiota contains more genes related to LPS synthesis and type III secretion systems, and lowered genes involved in central metabolism [110]. While the role of the intestinal microbiota in PD is not yet established, recent work shows that PD microbiota could transmit worsened motor defects to α-synuclein-overexpressing mice versus mice colonized with healthy control microbiota [73]. This suggests that the PD-associated microbiota may harbor microbes and functions that drive this neurodegenerative disease.
Mood and Behavioral Disorders
Anxiety
Manipulating the microbiota has been shown to influence neurobiology and behavior. Mice treated with high-dose broad-spectrum antibiotics show reduced anxiety-like behavior and increased exploratory activity as measured by the latency to step down from a pedestal and time spent in an illuminated compartment [111]. Even more intriguing, evidence suggests that host genetics can select for a microbiota that can modulate anxiety and behavior. Microbiota from anxiety-prone, timid BALB/c mice could inhibit the exploratory behavior of NIH Swiss Webster mice, and, conversely, brave Swiss Webster microbiota could increase the exploratory behavior when transferred to GF BALB/c mice. With both the antibiotic treatment and microbiota transfer, there were no changes in intestinal neurotransmitters (serotonin, noradrenaline, or dopamine) or markers of inflammation, and the signal was not conducted via the vagal nerve. However, increased exploratory behavior and reduced anxiety was associated with increases in brain-derived neurotrophic factor [111], indicating that the microbiota could regulate brain biochemistry.
Aggression
Sylvia et al. [112] demonstrated that treating male and female Siberian hamsters with enrofloxacin, a broad-spectrum fluoroquinolone antibiotic that does not cross the blood–brain barrier, reduced aggression in both male and female mice [112]. Of note, female mice were more sensitive to microbiota manipulation and showed reduced attacks after only a single 7-day treatment period and behavioral effects were sustained after antibiotics were stopped, whereas male mice required two 7-day treatment periods, and increased aggression returned after antibiotic cessation. Sex differences have been reported in the microbiome, and there is evidence that the microbiota can regulate testosterone [9], a hormone involved in aggressive behavior. Whether or not the mechanism of enrofloxacin is mediated by altered sex hormone signaling in this model is still unknown.
Autism
Over the past several decades, there has been a rise in the cases autism in the USA [113], and recent evidence suggests that the intestinal microbiota may play a role in this behavioral disorder. The first insight came from the observation that treatment with the antibiotic vancomycin could partially ameliorate behavioral problems in children with regressive autism [114]. Proinflammatory changes in GI tract may contribute to the pathogenesis. Individuals with autism have a higher prevalence of GI disorders, including inflammatory bowel disease [115], and gastrointestinal distress correlates with aggravated symptoms [116]. Maternal infection and inflammation during pregnancy has been linked to increasing the risk of autism. A mouse model of maternal immune activation (MIA) results in behavioral changes consistent with autism and has been utilized to explore the role of the intestinal microbiota in autism [117]. Offspring from MIA mothers had altered microbiota composition, including an increase in the families Lachnospiraceae, Porphyromonadaceae, and Prevotellaceae, and a decrease in Ruminococcaceae, Erysipelotrichaceae, and Alcaligenaceae. Characterizing GI function, MIA offspring have increased intestinal permeability, which may permit additional intestinal or microbial components to enter the circulation. Consistent with this concept of leaky gut, MIA offspring had altered serum metabolites, including the microbially derived uremic toxin 4-ethylphenylsulfate (4-EPS). Administration of 4-EPS resulted in increased anxiety-related behaviors in mice born to naïve mothers, demonstrating its role in modulating the gut–brain axis, although no changes were seen with other behaviors relating to sociability. Commensal microbiota play an active role in maintaining intestinal immune responses. Notably, Bacteroides fragilis, which contains polysaccharide A in its cell wall, can promote Treg cells and reduce intestinal inflammation [59, 118]. When MIA offspring were colonized with a polysaccharide A-positive B. fragilis strain, barrier function increased, alterations in microbiota composition normalized, and levels of 4-EPS in the serum dropped, which led to an improvement in behavior [117]. Altogether, these studies illustrate how imbalances in the microbiome driven by maternal immunity can lead to structural, chemical, and behavioral alterations in the offspring, which can be corrected by manipulating the microbiota.
Conclusion
Humans have co-evolved with the intestinal microbiota and this long-term relationship is maintained by regulatory factors that help select for a beneficial microbiota. The bidirectional immune, endocrine, and neural signaling pathways establish a mechanism for homeostatic communication between the microbiota and the CNS. The interaction between the brain and the intestinal microbiota plays an important role in mood, metabolism, cognition, and motor function. Disruptions in the microbiota–gut–brain axis can contribute to a variety of diseases, including anxiety, depression, MS, PD, and autism. Better understanding of which microbial components directly and indirectly signal to the brain could provide promising new targets for treating neurologic diseases.
References
Sender, R., S. Fuchs, and R. Milo. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell 2016; 164(3): p. 337-340.
Qin, J., R. Li, J. Raes, et al., A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 2010. 464(7285): p. 59-65.
Laukens, D., B.M. Brinkman, J. Raes, M. De Vos, and P. Vandenabeele, Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev., 2016. 40(1): p. 117-132.
Tremaroli, V. and F. Bäckhed, Functional interactions between the gut microbiota and host metabolism. Nature, 2012. 489(7415): p. 242-249.
Cho, I. and M.J. Blaser, The human microbiome: at the interface of health and disease. Nat Rev Genet, 2012. 13(4): p. 260-270.
Belkaid, Y. and O.J. Harrison, Homeostatic immunity and the microbiota. Immunity, 2017. 46(4): p. 562-576.
O'Hara, A. and F. Shanahan, The gut flora as a forgotten organ. EMBO Rep, 2006. 7(7): p. 688.
Collins, S.M., M. Surette, and P. Bercik, The interplay between the intestinal microbiota and the brain. Nature Rev Microbiol, 2012. 10(11): p. 735-742.
Markle, J.G.M., D.N. Frank, S. Mortin-Toth, et al., Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science, 2013. 339(6123): p. 1084-1088.
Sayin, S.I., A. Wahlström, J. Felin, et al., Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab, 2013. 17(2): p. 225-235.
Haiser, H.J. and P.J. Turnbaugh, Is it time for a metagenomic basis of therapeutics? Science, 2012. 336(6086): p. 1253-1255.
Yatsunenko, T., F.E. Rey, M.J. Manary, et al., Human gut microbiome viewed across age and geography. Nature, 2012. 486: p. 222-227.
Aagaard, K., J. Ma, K.M. Antony, et al., The placenta harbors a unique microbiome. Sci Transl Med 2014. 6(237): p. 237ra65-237ra65.
Wassenaar, T. and P. Panigrahi, Is a foetus developing in a sterile environment? Lett Appl Microbiol 2014. 59(6): p. 572-579.
Fardini, Y., P. Chung, R. Dumm, N. Joshi, and Y.W. Han, Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun, 2010. 78(4): p. 1789-1796.
Cox, L.M. and M.J. Blaser, Antibiotics in early life and obesity. Nat Rev Endocrinol, 2014. 11(3): p. 182-1920.
Dominguez-Bello, M.G., E.K. Costello, M. Contreras, et al., Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A, 2010. 107(26): p. 11971-11975.
Zeissig, S. and R.S. Blumberg, Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol, 2014. 15(4): p. 307-310.
Cox, L.M., S. Yamanishi, J. Sohn, et al., Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell, 2014. 158(4): p. 705-721.
Chung, H., S.J. Pamp, J.A. Hill, et al., Gut immune maturation depends on colonization with a host-specific microbiota. Cell, 2012. 149(7): p. 1578-1593.
Walker, W.A., Initial intestinal colonization in the human infant and immune homeostasis. Ann Nutr Metab, 2013. 63(s2): p. 8-15.
Borre, Y.E., G.W. O’Keeffe, G. Clarke, et al., Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 2014. 20(9): p. 509-518.
Stilling, R.M., T.G. Dinan, and J.F. Cryan, Microbial genes, brain & behaviour—epigenetic regulation of the gut–brain axis. Genes Brain Behav 2013. 13(1): p. 69-86.
Spor, A., O. Koren, and R. Ley, Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 2011. 9(4): p. 279-290.
Dąbrowska, K. and W. Witkiewicz, Correlations of host genetics and gut microbiome composition. Front Microbiol 2016. 7(1357).
Ridaura, V.K., J.J. Faith, F.E. Rey, et al., Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science, 2013. 341(6150): p. 1079.
Sommer, F. and F. Bäckhed, The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013. 11: p. 227.
Costello, E., C. Lauber, M. Hamady, et al., Bacterial community variation in human body habitats across space and time. Science, 2009. 326: p. 1694-1697.
Costello, E.K., K. Stagaman, L. Dethlefsen, B.J.M. Bohannan, and D.A. Relman, The application of ecological theory toward an understanding of the human microbiome. Science, 2012. 336(6086): p. 1255-1262.
David, L.A., C.F. Maurice, R.N. Carmody, et al., Diet rapidly and reproducibly alters the human gut microbiome. Nature, 2014. 505(7484): p. 559-563.
Ubeda, C. and E.G. Pamer, Antibiotics, microbiota, and immune defense. Trends Immunol, 2012. 33(9): p. 459-466.
Levy, M., A.A. Kolodziejczyk, C.A. Thaiss, and E. Elinav, Dysbiosis and the immune system. Nat Rev Immunol 2017; 17: 219-232.
Dethlefsen, L., S. Huse, M.L. Sogin, and D.A. Relman, The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLOS Biol, 2008. 6(11): p. e280.
Matamoros, S., C. Gras-Leguen, F. Le Vacon, G. Potel, and M.-F. de La Cochetiere, Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 2013. 21(4): p. 167-173.
Samuel, B.S., A. Shaito, T. Motoike, et al., Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A, 2008. 105(43): p. 16767-16772.
Clemmensen, C., T.D. Müller, S.C. Woods, et al., Gut–brain cross-talk in metabolic control. Cell, 2017. 168(5): p. 758-774.
Cox, L.M. and M.J. Blaser, Pathways in microbe-induced obesity. Cell Metab, 2013. 17(6): p. 883-894.
Cryan, J.F. and T.G. Dinan, Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012. 13(10): p. 701-712.
Foster, J.A. and K.-A.M. Neufeld, Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci, 2013. 36(5): p. 305-312.
Bravo, J.A., P. Forsythe, M.V. Chew, et al., Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011. 108(38): p. 16050-16055.
Bercik, P., A.J. Park, D. Sinclair, et al., The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil 2011. 23(12): p. 1132-1139.
Kunze, W.A., Y.K. Mao, B. Wang, et al., Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 2009. 13(8b): p. 2261-2270.
Bellono, N.W., J.R. Bayrer, D.B. Leitch, et al., Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell, 2017. 170(1): p. 185-198. e16.
Brierley, S.M. and D.R. Linden, Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014. 11(10): p. 611-627.
Farup, P.G., K. Rudi, and K. Hestad, Faecal short-chain fatty acids-a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol 2016. 16(1): p. 51.
Tanaka, K., M. Budd, M. Efron, and K. Isselbacher, Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc Natl Acad Sci U S A 1966. 56(1): p. 236-242.
Fujinaga, Y., Y. Sugawara, and T. Matsumura. Uptake of botulinum neurotoxin in the intestine, in botulinum neurotoxins, A. Rummel and T. Binz, Editors. 2013, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 45–59.
Brown, N. and S. Desai, Infantile botulism: a case report and review. J Emerg Med 2013. 45(6): p. 842-845.
Sudo, N., Y. Chida, Y. Aiba, et al., Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 2004. 558(1): p. 263-275.
Webster Marketon, J. and E. Sternberg, The glucocorticoid receptor: a revisited target for toxins. Vol. 2. 2010. 1357-80.
Reichardt, H.M., T. Umland, A. Bauer, O. Kretz, and G. Schütz, Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol 2000. 20(23): p. 9009-9017.
Wang, Y. and L.H. Kasper, The role of microbiome in central nervous system disorders. Brain Behav Immun 2014. 38(C): p. 1-12.
Ichimura, A., A. Hirasawa, T. Hara, and G. Tsujimoto, Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat, 2009. 89: p. 82-8.
Honda, K. and D.R. Littman, The microbiota in adaptive immune homeostasis and disease. Nature, 2016. 535(7610): p. 75-84.
Kamada, N., S.-U. Seo, G.Y. Chen, and G. Núñez, Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol, 2013. 13(5): p. 321-335.
Ivanov, I.I., K. Atarashi, N. Manel, et al., Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell, 2009. 139(3): p. 485-498.
Ivanov, I.I. and D.R. Littman, Segmented filamentous bacteria take the stage. Mucosal Immunol 2010: p. 209–212.
Atarashi, K.,T. Tanoue, T. Shima, et al., Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 2011. 331(6015): p. 337-341.
Round, J.L. and S.K. Mazmanian, Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci 2010. 107(27): p. 12204-12209.
Blaser, M.J. and J.C. Atherton, Helicobacter pylori persistence: biology and disease. J Clin Invest, 2004. 113(3): p. 321-333.
Robinson, K., R.H. Argent, and J.C. Atherton, The inflammatory and immune response to Helicobacter pylori infection. Best Pract Res Clin Gastroenterol 2007. 21(2): p. 237-259.
Parsot, C. and P.J. Sansonetti, The virulence plasmid of Shigellae: an archipelago of pathogenicity islands?, in Pathogenicity islands and other mobile virulence elements. 1999, American Society of Microbiology. p. 151-165.
Kaper, J.B., J.L. Mellies, and J.P. Nataro, Pathogenicity islands and other mobile genetic elements of diarrheagenic Escherichia coli, in Pathogenicity islands and other mobile virulence elements. 1999, American Society of Microbiology. p. 33-58.
Hill, K.K., G. Xie, B.T. Foley, and T.J. Smith, Genetic diversity within the botulinum neurotoxin-producing bacteria and their neurotoxins. Toxicon, 2015. 107(Part A): p. 2-8.
Montecucco, C. and M.B. Rasotto, On botulinum neurotoxin variability. MBio, 2015. 6(1): p. e02131-14.
Louis, P. and H.J. Flint, Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017. 19(1): p. 29-41.
Wong, J.M.W., R. de Souza, C.W.C. Kendall, A. Emam, and D.J.A. Jenkins, Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol, 2006. 40(3): p. 235-43.
Koh, A., F. De Vadder, P. Kovatcheva-Datchary, and F. Bäckhed, From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell, 2016. 165(6): p. 1332-1345.
Louis, P. and H.J. Flint, Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett, 2009. 294(1): p. 1-8.
Erny, D., A.L.H. de Angelis, D. Jaitin, et al., Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015. 18(7): p. 965-977.
Chang, P.V., L. Hao, S. Offermanns, and R. Medzhitov, The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci, 2014. 111(6): p. 2247-2252.
Singh, N., A. Gurav, S. Sivaprakasam, et al., Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity, 2014. 40(1): p. 128-139.
Sampson, T.R., J.W. Debelius, T. Thron, et al., Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 2016. 167(6): p. 1469-1480. e12.
Prinz, M. and J. Priller, Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014. 15(5): p. 300-312.
Rothhammer, V., I.D. Mascanfroni, L. Bunse, et al., Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and CNS inflammation via the aryl hydrocarbon receptor. Nat Med, 2016. 22(6): p. 586.
Rothhammer, V. and F.J. Quintana, Environmental control of autoimmune inflammation in the central nervous system. Curr Opin Immunol, 2016. 43: p. 46-53.
Myint, A.-M., Y.K. Kim, R. Verkerk, et al., Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007. 98(1): p. 143-151.
Clarke, G., S. Grenham, P. Scully, et al., The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry, 2013. 18(6): p. 666.
Desbonnet, L., G. Clarke, A. Traplin, et al., Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun, 2015. 48: p. 165-173.
Berer, K., M. Mues, M. Koutrolos, et al., Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature, 2011. 479(7374): p. 538.
Lee, Y.K., J.S. Menezes, Y. Umesaki, and S.K. Mazmanian, Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A, 2011. 108(Suppl. 1): p. 4615-4622.
Ochoa-Reparaz, J., D.W. Mielcarz, L.E. Ditrio, et al., Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol 2009. 183(10): p. 6041-6050.
Yokote, H., S. Miyake, J.L. Croxford, et al., NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol 2010. 173(6): p. 1714-1723.
Colpitts, S.L., E.J. Kasper, A. Keever, et al., A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes, 2017. 137(5): p. 561-573.
Alonso, A., Antibiotic use and risk of multiple sclerosis. Am J Epidemiol, 2006. 163(11): p. 997-1002.
Norgaard, M., R.B. Nielsen, J.B. Jacobsen, et al., Use of penicillin and other antibiotics and risk of multiple sclerosis: a population-based case-control study. Am J Epidemiol, 2011. 174(8): p. 945-948.
Ren, J., H. Ni, M. Kim, et al., Allergies, antibiotics use, and multiple sclerosis. Curr Med Res Opin 2017. 167: p. 1-6.
Jangi, S., R. Gandhi, L.M. Cox, et al., Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016. 7: p. 12015.
Miyake, S., S. Kim, W. Suda, et al., Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLOS ONE, 2015. 10(9): p. e0137429.
Cantarel, B.L., E. Waubant, C. Chehoud, et al., Gut microbiota in multiple sclerosis. J Invest Med, 2015. 63(5): p. 729-734.
Chen, J., N. Chia, K.R. Kalari, et al., Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016. 6.
van Passel, M.W.J., R. Kant, E.G. Zoetendal, et al., The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLOS ONE, 2011. 6(3): p. e16876.
Everard, A., C. Belzer, L. Geurts, et al., Crosstalk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A, 2013. 110(22): p. 9066-9071.
Derrien, M., E.E. Vaughan, C.M. Plugge, and W.M. de Vos, Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Micr, 2004. 54(5): p. 1469-1476.
Dubourg, G., F. Cornu, S. Edouard, et al., First isolation of Akkermansia muciniphila in a blood-culture sample. Clin Microbiol Infect 2017;23:682-683.
Blais Lecours, P., C. Duchaine, M. Taillefer, et al., Immunogenic properties of archaeal species found in bioaerosols. PLOS ONE, 2011. 6(8): p. e23326.
Verma, R., A.K. Verma, V. Ahuja, and J. Paul, Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol 2010. 48(11): p. 4279-4282.
Samuel, B.S., E.E. Hansen, J.K. Manchester, et al., Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci U S A 2007. 104(25): p. 10643-10648.
Yamabe, K., H. Maeda, S. Kokeguchi, et al., Distribution of Archaea in Japanese patients with periodontitis and humoral immune response to the components. FEMS Microbiol Lett 2008. 287(1): p. 69-75.
Krishnan, L., S. Sad, G.B. Patel, and G.D. Sprott, The potent adjuvant activity of archaeosomes correlates to the recruitment and activation of macrophages and dendritic cells in vivo. J Immunol 2001. 166(3): p. 1885-1893.
Berer, K., L.A. Gerdes, E. Cekanaviciute, et al., Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A, 2017. 114(40): p. 10719-10724.
Cekanaviciute, E., B.B. Yoo, T.F. Runia, et al., Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A, 2017: p. 201711235.
Fasano, A., N.P. Visanji, L.W. Liu, A.E. Lang, and R.F. Pfeiffer, Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol 2015. 14(6): p. 625-639.
Houser, M.C. and M.G. Tansey, The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinson's Disease, 2017. 3: p. 1.
Chen, H., E.J. Zhao, W. Zhang, et al., Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener, 2015. 4(1): p. 1.
Shannon, K.M., A. Keshavarzian, H.B. Dodiya, S. Jakate, and J.H. Kordower, Is alpha-synuclein in the colon a biomarker for premotor Parkinson's Disease? Evidence from 3 cases. Mov Disord 2012. 27(6): p. 716-719.
Unger, M.M., J. Spiegel, K.-U. Dillmann, et al., Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord 2016. 32: p. 66-72.
Hasegawa, S., S. Goto, H. Tsuji, et al., Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLOS ONE, 2015. 10(11): p. e0142164.
Scheperjans, F., V. Aho, P.A. Pereira, et al., Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015. 30(3): p. 350-358.
Keshavarzian, A., S.J. Green, P.A. Engen, et al., Colonic bacterial composition in Parkinson's disease. Mov Disord 2015. 30(10): p. 1351-1360.
Bercik, P., E. Denou, J. Collins, et al., The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 2011. 141(2): p. 599-609.e3.
Sylvia, K.E., C.P. Jewell, N.M. Rendon, E.A. St. John, and G.E. Demas, Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav Immun 2017. 60(Supplement C): p. 51-62.
Autism and Development Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ 2012;61:1-19.
Sandler, R.H., S.M. Finegold, E.R. Bolte, et al., Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000. 15(7): p. 429-435.
Kohane, I.S., A. McMurry, G. Weber, et al., The co-morbidity burden of children and young adults with autism spectrum disorders. PLOS ONE, 2012. 7(4): p. e33224.
Adams, J.B., L.J. Johansen, L.D. Powell, D. Quig, and R.A. Rubin, Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol, 2011. 11(1): p. 22.
Hsiao, E.Y., S.W. McBride, S. Hsien, et al., Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell, 2013: p. 1451-63.
Mazmanian, S.K., J.L. Round, and D.L. Kasper, A microbial symbiosis factor prevents intestinal inflammatory disease. Nature, 2008. 453(7195): p. 620.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 506 kb)
Rights and permissions
About this article
Cite this article
Cox, L.M., Weiner, H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics 15, 135–145 (2018). https://doi.org/10.1007/s13311-017-0598-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-017-0598-8