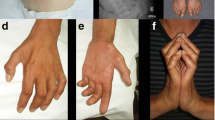
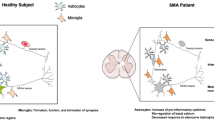
Abstract
Spinal muscular atrophy (SMA) is a frequently fatal neuromuscular disorder and the most common inherited cause of infant mortality. SMA results from reduced levels of the survival of motor neuron (SMN) protein. Although the disease was first described more than a century ago, a precise understanding of its genetics was not obtained until the SMA genes were cloned in 1995. This was followed in rapid succession by experiments that assigned a role to the SMN protein in the proper splicing of genes, novel animal models of the disease, and the eventual use of the models in the pre clinical development of rational therapies for SMA. These successes have led the scientific and clinical communities to the cusp of what are expected to be the first truly promising treatments for the human disorder. Yet, important questions remain, not the least of which is how SMN paucity triggers a predominantly neuromuscular phenotype. Here we review how our understanding of the disease has evolved since the SMA genes were identified. We begin with a brief description of the genetics of SMA and the proposed roles of the SMN protein. We follow with an examination of how the genetics of the disease was exploited to develop genetically faithful animal models, and highlight the insights gained from their analysis. We end with a discussion of ongoing debates, future challenges, and the most promising treatments to have emerged from our current knowledge of the disease.


Similar content being viewed by others
References
Werdnig G. Zwei frühinfantile hereditäre Fälle von progressiver Muskelatrophie unter dem Bilde der Dystrophie, aber auf neurotischer Grundlage. Arch Psychiat Nervenkr 1891;22:437-480.
Hoffmann J. Ueber chronische spinale Muskelatrophie im Kindesalter, auf familiärer Basis. Deutsch Z Nervenheilk 1893; 3:427-470.
Hoffmann J. Weiterer Beitrag zur Lehre von der hereditären progressiven spinalen Muskelatrophie im Kindesalter nebst Bemerkungen über den fortschreitenden Muskelschwund im Allgemeinen . Deutsch Z Nervenheilk 1897;10:292-320.
Hoffmann J. Dritter Beitrag zur Lehre von der hereditären progressiven spinalen Muskelatrophie im Kindesalter. Deutsch Z Nervenheilk 1900;18:217-224.
Kugelberg E, Welander L. Heredofamilial juvenile muscular atrophy simulating muscular dystrophy. AMA Arch Neurol Psychiatry 1956;75:500-509.
Dubowitz V. Infantile muscular atrophy. A prospective study with particular reference to a slowly progressive variety. Brain 1964;87:707-718.
Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis 1996;3:97-110.
Simic G, Mladinov M, Seso Simic D, et al. Abnormal motoneuron migration, differentiation, and axon outgrowth in spinal muscular atrophy. Acta Neuropathol 2008;115:313-326.
Chou S, Nonaka, I. Werdnig-Hoffmann disesase: proposal of a pathogenic mechanism. Acta Neuropathol 1978;41:45-54.
Marshal A, Duchen, LW. Sensory involvement in infantile spinal muscular atrophy. J Neurol Sci 1975;26:349-359.
Towfighi J, Young, RSK, Ward, RM. Is Werdnig-Hoffmann disease a pure lower motor neuron disorder? Acta Neuropathol 1985;65:270-280.
Peress NS, Stermann AB, Miller R, Kaplan CG, Little BW. “Chromatolytic” neurons in lateral geniculate body in Werdnig-Hoffmann disease. Clin Neuropathol 1986;5:69-72.
Murayama TL, Bouldin TW, Suzuki K. Immunocytochemcial and ultrastructural studies of Werdnig-Hoffmann disease. Acta Neuropathol 1991;81:408-417.
Dubowitz V. Muscle disorders in childhood, 2nd ed. Saunders, Philadelphia, PA, 1995.
Nadeau A, D'Anjou G, Debray G, et al. A newborn with spinal muscular atrophy type 0 presenting with a clinicopathological picture suggestive of myotubular myopathy. J Child Neurol 2007;22:1301-1304.
Schmid A, DiDonato CJ. Animal models of spinal muscular atrophy. J Child Neurol 2007; 22:1004-1012.
Murray LM, Comley LH, Thomson D, et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet 2008;17:949-962.
Kariya S, Park GH, Maeno-Hikichi Y, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet 2008;17:2552-2569.
Ling KK, Lin MY, Zingg B, Feng Z, Ko CP. Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS One 2010;5: e15457.
Martinez-Hernandez R, Bernal S, Also-Rasso E, et al. Synaptic defects in type 1 spinal muscular atrophy in human development. J Pathol 2013;229:49-61.
Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med 2013;19:40-50.
Bowerman M, Swoboda KJ, Michalski JP, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol 2012;72:256-268.
Sugarman EA, Nagan N, Zhu H, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet 2012;20:27-32.
Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155-165.
Monani UR, Lorson, CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet 1999;8:1177-1183.
Kashima T, Manley JL. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat Genet 2003;34:460-463.
Lorson CL, Hahnen E, Androphy EJ, and Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 1999;96:6307-6311.
Cho S, Dreyfuss G. A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity. Genes Dev 2010;24:438-442.
Carpten JD, DiDonato CJ, Ingraham SE, et al. A YAC contig of the region containing the spinal muscular atrophy gene (SMA): identification of an unstable region. Genomics 1994; 24:351-356.
Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet 1997;6:1205-1214.
Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 1997;16:265-269.
Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet 2002;70:358-368.
Battaglia G, Princivalle A, Forti F, Lizier C, Zeviani M. Expression of the SMN gene, the spinal muscular atrophy determining gene, in the mammalian central nervous system. Hum Mol Genet 1997;6:1961-1971.
Liu Q, Dreyfuss G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J 1996;15:3555-3565.
Liu Q, Fischer U, Wang F, Dreyfuss G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 1997;90:1013-1021.
Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 1997;90:1023-1029.
Sumpter V, Kahrs A, Fischer U, Kornstadt U, Luhrmann R. In vitro reconstitution of U1 and U2 snRNPs from isolated proteins and snRNA. Mol Biol Rep 1992;16:229-240.
Raker VA, Hartmuth K, Kastner B, Luhrmann R. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol Cell Biol 1999;19:6554-6565.
Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science 2002;298:1775-1779.
Battle DJ, Kasim M, Yong J, et al. The SMN complex: an assembly machine for RNPs. Cold Spring Harb Symp Quant Biol 2006;71:313-320.
Gabanella F, Butchbach ME, Saieva L, et al. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS One 2007;2:e921.
Zhang Z, Lotti F, Dittmar K, et al. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 2008;133:585-600.
See K, Yadav P, Giegerich M, et al. SMN deficiency alters Nrxn2 expression and splicing in zebrafish and mouse models of spinal muscular atrophy. Hum Mol Genet 2014;23:1754-1770.
Lotti F, Imlach WL, Saieva L, et al. An SMN-dependent U12 splicing event essential for motor circuit function. Cell 2012;151:440-454.
Wishart TM, Mutsaers CA, Riessland M, et al. Dysregulation of ubiquitin homeostasis and β-catenin signaling promote spinal muscular atrophy. J Clin Invest 2014;124:1821-1834.
Zhang Z, Pinto AM, Wan L, et al. Dysregulation of synaptogenesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proc Natl Acad Sci 2013;110:19348-19353.
Zhang H, Xing L, Rossoll W, Wichterle H, Singer RH, Bassell GJ. Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons. J Neurosci 2006;26:8622-8632.
Terns MP, Terns RM. Macromolecular complexes: SMN—the master assembler. Curr Biol 2001;11:862-864.
Akten B, Kye MJ, Hao le T, et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc Natl Acad Sci USA 2011;108:10337-10342.
Hubers L, Valderrama-Carvajal H, Laframboise J, Timbers J, Sanchez G, Côté J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum Mol Genet 2011;20:553-579.
Tadesse H, Deschênes-Furry J, Boisvenue S, Côté J. KH-type splicing regulatory protein interacts with survival motor neuron protein and is misregulated in spinal muscular atrophy. Hum Mol Genet 2008;17:506-524.
Rossoll W, Kröning AK, Ohndorf UM, Steegborn C, Jablonka S, Sendtner M. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum Mol Genet 2002;11:93-105.
Carrel TL, McWhorter ML, Workman E, et al. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J Neurosci 2006;26:11014-11022.
Praveen K, Wen Y, Matera AG. A Drosophila model of spinal muscular atrophy uncouples snRNP biogenesis functions of survival motor neuron from locomotion and viability defects. Cell Rep 2012;1:624-631.
Tisdale S, Lotti F, Saieva L, et al. SMN is essential for the biogenesis of U7 small nuclear ribonucleoprotein and 3'-end formation of histone mRNAs. Cell Rep 2013;5:1187-1195.
Rochette CF, Gilbert N, Simard LR. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Genet 2001;108:255-256.
DiDonato CJ, Chen XN, Noya D, Korenberg JR, Nadeau JH, Simard LR. Cloning, characterization, and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res 1997;7:339-352.
Viollet L, Bertrandy S, Bueno Brunialti AL, et al. cDNA isolation, expression, and chromosomal localization of the mouse survival motor neuron gene (Smn). Genomics 1997;40:185-188.
Schrank B, Götz R, Gunnersen JM, et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci U S A 1997;94:9920-9925.
Monani UR, Sendtner M, Coovert DD, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn (-/-) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet 2000;9:333-339.
Hsieh-Li HM, Chang JG, Jong YJ, et al. A mouse model for spinal muscular atrophy. Nat Genet 2000;24:66-70.
Le TT, Pham LT, Butchbach ME, et al. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet 2005;14 845-857.
Monani UR, Pastore MT, Gavrilina TO, et al. A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy. J Cell Biol 2003;162:919-931.
Osborne M, Gomez D, Feng Z, et al. Characterization of behavioral and neuromuscular junction phenotypes in a novel allelic series of SMA mouse models. Hum Mol Genet 2012;21:4431-4447.
Michaud M, Arnoux T, Bielli S, et al. Neuromuscular defects and breathing disorders in a new mouse model of spinal muscular atrophy. Neurobiol Dis 2010;38:125-135.
Hammond SM, Gogliotti RG, Rao V, Beauvais A, Kothary R, DiDonato CJ. Mouse survival motor neuron alleles that mimic SMN2 splicing and are inducible rescue embryonic lethality early in development but not late. PLoS One 2010;5:e15887.
Gavrilina TO, McGovern VL, Workman E, et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet 2008;17:1063-1075.
Park GH, Maeno-Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci 2010;30:12005-12019.
Gogliotti RG, Quinlan KA, Barlow CB, et al. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci 2012;32:3818-3829.
Martinez TL, Kong L, Wang X, et al. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci 2012;32:8703-8715.
Lee AJ, Awano T, Park GH, Monani UR. Limited phenotypic effects of selectively augmenting the SMN protein in the neurons of a mouse model of severe spinal muscular atrophy. PLoS One 2012;7:e46353.
Shababi M, Lorson CL, Rudnik-Schöneborn SS. Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? J Anat 2014;224:15-28.
Hua Y, Sahashi K, Rigo F, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011;478:123-126.
Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science 2007;104:2709-2714.
McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science 2011;833:186.
Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 2000;101:57-66.
Kordasiewicz HB, Stanek LM, Wancewicz EV, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron 2012;74:1031-1044.
Le TT, McGovern VL, Alwine IE, et al. Temporal requirement for high SMN expression in SMA mice. Hum Mol Genet 2011;20:3578-3591.
Lutz CM, Kariya S, Patruni S, et al. Post-symptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J Clin Invest 2011;8:3029-3041.
Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotech 2010;28:271-274.
Hao le T, Duy PQ, Jontes JD, et al. Temporal requirement for SMN in motoneuron development. Hum Mol Genet 2013;22:2612-2625.
Kariya S, Obis T, Garone C, et al. Requirement of enhanced Survival Motoneuron protein imposed during neuromuscular junction maturation. J Clin Invest 2014;124:785-800.
Gabanella F, Carissimi C, Usiello A, Pellizzoni. The activity of the spinal muscular atrophy protein is regulated during development and cellular differentiation. Hum Mol Genet 2005; 14:3629-3642.
Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology 2007;69:1931-1936.
Montes J, McIsaac TL, Dunaway S, et al. Falls and spinal muscular atrophy: exploring cause and prevention. Muscle Nerve 2013;47:118-123.
Echaniz-Laguna A, Bousiges O, Loeffler JP, Boutillier AL. Histone deacetylase inhibitors: therapeutic agents and research tools for deciphering motor neuron diseases. Curr Med Chem 2008;15:1263-1273.
Chen TH, Chang JG, Yang YH, et al. Randomized, double-blind, placebo-controlled trial of hydroxyurea in spinal muscular atrophy. Neurology 2010;75:2190-2197.
Mercuri E, Bertini E, Messina S, et al. Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromusc Disord 2004;14:130-135.
Mercuri E, Bertini E, Messina S, et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology 2007;68:51-55.
Swoboda KJ, Scott CB, Reyna SP, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS One 2009;4:e5268.
Avila AM, Burnett BG, Taye AA, et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest 2007;117:659-671
Garbes L, Riessland M, Hölker I, et al. LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate. Hum Mol Genet 2009;18:3645-3658.
Kwon DY, Motley WW, Fischbeck KH, Burnett BG. Increasing expression and decreasing degradation of SMN ameliorate the spinal muscular atrophy phenotype in mice. Hum Mol Genet 2011;20:3667-3677.
Naryshkin N, Narasimhan J, Dakka A, et al. Small molecule compounds correct alternative splicing of the SMN2 gene and restore SMN protein expression and function. Neuromusc Dis 2012;22:848.
Butchbach ME, Singh J, Thorsteinsdóttir M, et al. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum Mol Genet 2010;19:454-467.
Hua Y, Sahashi K, Rigo F, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011;478:123-126.
Porensky PN, Mitrpant C, McGovern, et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum Mol Genet 2012;21:1625-1638.
Rigo F, Chun SJ, Norris DA, et al. Pharmacology of a central nervous system delivered 2’-o-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther 2014;350:46-55.
Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Delivery of a read-through inducing compound, TC007, lessens the severity of a spinal muscular atrophy animal model. Hum Mol Genet 2009;18:3906-3913.
Bordet T, Buisson B, Michaud M, et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther 2007;322:709-720.
Bowerman M, Murray LM, Boyer JG, Anderson CL, Kothary R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med 2012;10:24
Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotech 2009;27:59-65.
Valori CF, Ning K, Wyles M, et al. Systemic Delivery of scAAV9 Expressing SMN Prolongs Survival in a Model of Spinal Muscular Atrophy. Sci Transl Med 2010; 2:35ra42.
Dominguez E, Marais T, Chatauret N, et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet 2011;20:681-693.
Gowing G, Svendsen, CN. Stem cell transplantation for motor neuron disease: current approaches and future perspectives. Neurotherapeutics 2011;8:591-606.
Corti S, Nizzardo M, Nardini M, et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J Clin Invest 2008;118:3316-3330.
Yamazaki T, Chen S, Yu Y, et al. FUS-SMN protein interactions link the motor neuron diseases ALS and SMA. Cell Rep 2012;2:799-806.
Kariya S, Re D, Jacquier A, et al. Mutant superoxide dismutase 1 (SOD1), a cause of amyotrophic lateral sclerosis, disrupts the recruitment of SMN, the spinal muscular atrophy protein to nuclear Cajal bodies. Hum Mol Genet 2012;21:3421-3434.
Tsuiji H, Iguchi Y, Furuya A, et al. Spliceosome integrity is defective in the motor neuron diseases ALS and SMA. EMBO Mol Med 2013;5:221-234.
Acknowledgments
We are grateful to Drs. S. Kariya and R. Finkel for the data presented in Fig. 1. We apologize to those of our colleagues whose original work could not be directly cited in the review. Research on SMA in the Monani laboratory is supported by MDA-USA, SMA-Europe, the Motor Neuron Center, Columbia University, Department of Defense (W81XWH-11-1-0753), and National Institutes of Health R01NS057482.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1224 kb)
Rights and permissions
About this article
Cite this article
Awano, T., Kim, JK. & Monani, U.R. Spinal Muscular Atrophy: Journeying From Bench to Bedside. Neurotherapeutics 11, 786–795 (2014). https://doi.org/10.1007/s13311-014-0293-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-014-0293-y