Abstract
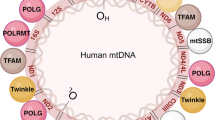
Mitochondrial DNA (mtDNA) depletion syndromes (MDS) are a genetically and clinically heterogeneous group of autosomal recessive disorders that are characterized by a severe reduction in mtDNA content leading to impaired energy production in affected tissues and organs. MDS are due to defects in mtDNA maintenance caused by mutations in nuclear genes that function in either mitochondrial nucleotide synthesis (TK2, SUCLA2, SUCLG1, RRM2B, DGUOK, and TYMP) or mtDNA replication (POLG and C10orf2). MDS are phenotypically heterogeneous and usually classified as myopathic, encephalomyopathic, hepatocerebral or neurogastrointestinal. Myopathic MDS, caused by mutations in TK2, usually present before the age of 2 years with hypotonia and muscle weakness. Encephalomyopathic MDS, caused by mutations in SUCLA2, SUCLG1, or RRM2B, typically present during infancy with hypotonia and pronounced neurological features. Hepatocerebral MDS, caused by mutations in DGUOK, MPV17, POLG, or C10orf2, commonly have an early-onset liver dysfunction and neurological involvement. Finally, TYMP mutations have been associated with mitochondrial neurogastrointestinal encephalopathy (MNGIE) disease that typically presents before the age of 20 years with progressive gastrointestinal dysmotility and peripheral neuropathy. Overall, MDS are severe disorders with poor prognosis in the majority of affected individuals. No efficacious therapy is available for any of these disorders. Affected individuals should have a comprehensive evaluation to assess the degree of involvement of different systems. Treatment is directed mainly toward providing symptomatic management. Nutritional modulation and cofactor supplementation may be beneficial. Liver transplantation remains controversial. Finally, stem cell transplantation in MNGIE disease shows promising results.

Similar content being viewed by others
References
Moraes CT, Shanske S, Tritschler HJ, et al. MtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet 1991;48:492–501.
Sarzi E, Bourdon A, Chrétien D, et al. Mitochondrial DNA depletion is a prevalent cause of multiple respiratory chain deficiency in childhood. J Pediatr 2007;150:531–534.
Spinazzola A, Invernizzi F, Carrara F, et al. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis 2009;32:143–158.
Suomalainen A, Isohanni P. Mitochondrial DNA depletion syndromes—many genes, common mechanisms. Neuromuscul Disord 2010;20:429–437.
Spinazzola A. Mitochondrial DNA mutations and depletion in pediatric medicine. Semin Fetal Neonatal Med 2011;16:190–196.
Johansson M, Karlsson A. Cloning of the cDNA and chromosome localization of the gene for human thymidine kinase 2. J Biol Chem 1997;272:8454–8458.
Johansson M, Karlsson A. Cloning and expression of human deoxyguanosine kinase cDNA. Proc Nat Acad Sci 1996;93:7258–7262.
Kowluru A, Tannous M, Chen HQ. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys 2002;398:160–169.
Pontarin G, Fijolek A, Pizzo P, et al. Ribonucleotide reduction is a cytosolic process in mammalian cells independently of DNA damage. Proc Natl Acad Sci 2008;105:17801–17806.
Lecrenier N, van der Bruggen P, Foury F. Mitochondrial DNA polymerases from yeast to man: a new family of polymerases. Gene 1997;185:147–152.
Spelbrink JN, Li FY, Tiranti V, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet 2001;28:223–231.
Dallabona C, Marsano RM, et al. Sym1, the yeast ortholog of the MPV17 human disease protein, is a stress-induced bioenergetic and morphogenetic mitochondrial modulator. Hum Mol Genet 2010;19:1098–1107.
Pons R, Andreetta F, Wang CH, et al. Mitochondrial myopathy simulating spinal muscular atrophy. Pediatr Neurol 1996;15:153–158.
Galbiati S, Bordoni A, Papadimitriou D, et al. New mutation in TK2 gene associated with mitochondrial DNA depletion. Pediatr Neurol 2006; 34:177–185.
Oskoui M, Davidzon G, Pascual J, et al. Clinical spectrum of mitochondrial DNA depletion due to mutation in the thymidine kinase 2 gene. Arch Neurol 2006;63:1122–1126.
Blakely E, He L, Gardner JL, et al. Novel mutationin the TK2 gene associated with fatal mitochondrial DNA depletion myopathy. Neuromuscul Disord 2008;18:557–560.
Gotz A, Isohanni P, Pihko H, et al. Thymidine kinase 3 defects can cause multi-tissue mtDNA depletion syndrome. Brain 2008;131:2841–2850.
Collins J, Bove KE, Dimmock D, Morehart P, Wong LJ, Wong B. Progressive myofiber loss with extensive fibro-fatty replacement in a child with mitochondrial DNA depletion syndrome and novel thymidine kinase 2 gene mutations. Neuromuscul Disord 2009;19:784–787.
Zhang S, Li FY, Bass HN, et al. Application of oligonucleotide array CGH to the simultaneous detection of a deletion in the nuclear TK2 gene and mtDNA depletion. Mol Genet Metab 2010;99:53–57.
Lesko N, Naess K, Wibom R, et al. Two novel mutations in thymidine kinase-2 cause early onset fatal encephalomyopathy and severe mtDNA depletion. Neuromuscul Disord 2010;20:198–203.
Martí R, Nascimento A, Colomer J, et al. Hearing loss in a patient with the myopathic form of mitochondrial DNA depletion syndrome and novel mutation in the TK2 gene. Pediatr Res 2010;68:151–154.
Behim A, Jardel C, Claeys KG, et al. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology 2012;78:644–648.
Tyynismaa H, Sun R, Ahola-Erkkilä S, et al. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum Mol Genet 2012;21:66–75.
Elpeleg O, Miller C, Hershkovitz E, et al. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet 2005;76:1081–1086.
Carrozzo R, Dionisi-Vici C, Steuerwald U, et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 2007;130:862–874.
Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 2007;130:853–561.
Ostergaard E, Christensen E, Kristensen E, et al. Deficiency of the alpha subunit of succinate-coenzyme A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am J Hum Genet 2007;81:383–387.
Morava E, Steuerwald U, Carrozzo R, et al. Dystonia and deafness due to SUCLA2 defect; Clinical course and biochemical markers in 16 children. Mitochondrion 2009;9:438–442.
Ostergaard E. SUCLA2-related mitochondrial DNA depletion syndrome, encephalomyopathic form, with mild methylmalonic aciduria. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK6803/. Updated 26 May 2009. Accessed 14 Nov 2012.
Ostergaard E, Schwartz M, Batbayli M, et al. A novel missense mutation in SUCLG1 associated with mitochondrial DNA depletion, encephalomyopathic form, with methylmalonic aciduria. Eur J Pediatr 2010;169:201–205.
Bourdon A, Minai L, Serre V, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 2007;39:776–780.
Bornstein B, Area E, Flanigan KM, et al. Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene. Neuromuscul Disord 2008;18:453–459.
Acham-Roschitz B, Plecko B, Lindbichler F, et al. A novel mutation of the RRM2B gene in an infant with early fatal encephalomyopathy, central hypomyelination, and tubulopathy. Mol Genet Metab 2009;98:300–304.
Kollberg G, Darin N, Benan K, et al. A novel homozygous RRM2B missense mutation in association with severe mtDNA depletion. Neuromuscul Disord 2009;19:147–150.
Shaibani A, Shchelochkov OA, Zhang S, et al. Mitochondrial neurogastrointestinal encephalopathy due to mutations in RRM2B. Arch Neurol 2009;66:1028–1032.
Tyynismaa H, Ylikallio E, Patel M, Molnar MJ, Haller RG, Suomalainen A. A heterozygous truncating mutation in RRM2B causes autosomal-dominant progressive external ophthalmoplegia with multiple mtDNA deletions. Am J Hum Genet 2009;85:290–295.
Fratter C, Raman P, Alston CL, et al. RRM2B mutations are frequent in familial PEO with multiple mtDNA deletions. Neurology 2011;76:2032–2034.
Scaglia F, Dimmock D, Wong LJ. DGUOK-related mitochondrial DNA depletion syndrome, hepatocerebral form. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK7040/. Updated 18 Jun 2009. Accessed 14 Nov 2012.
Ducluzeau PH, Lachaux A, Bouvier R, Streichenberger N, Stepien G, Mousson B. Depletion of mitochondrial DNA associated with infantile cholestasis and progressive liver fibrosis. J Hepatol 1999;30:149–155.
Mandel H, Szargel R, Labay V, et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet 2001;29:337–341.
Salviati L, Sacconi S, Mancuso M, et al. Mitochondrial DNA depletion and dGK gene mutations. Ann Neurol 2002;52:311–317.
Taanman JW, Kateeb I, Muntau AC, Jaksch M, Cohen N, Mandel H. A novel mutation in the deoxyguanosine kinase gene causing depletion of mitochondrial DNA. Ann Neurol 2002;52:237–239.
Filosto M, Mancuso M, Tomelleri G, et al. Hepato-cerebral syndrome: genetic and pathological studies in an infant with a dGK mutation. Acta Neuropathol 2004;108:168–171.
Rabinowitz SS, Gelfond D, Chen CK, et al. Hepatocerebral mitochondrial DNA depletion syndrome: clinical and morphologic features of a nuclear gene mutation. J Pediatr Gastroenterol Nutr 2004;38:216–220.
Labarthe F, Dobbelaere D, Devisme L, et al. Clinical, biochemical and morphological features of hepatocerebral syndrome with mitochondrial DNA depletion due to deoxyguanosine kinase deficiency. J Hepatol 2005;43:333–341.
Mancuso M, Ferraris S, Pancrudo J,et al. New DGK gene mutations in the hepatocerebral form of mitochondrial DNA depletion syndrome. Arch Neurol 2005;62:745–747.
Slama A, Giurgea I, Debrey D, et al. Deoxyguanosine kinase mutations and combined deficiencies of the mitochondrial respiratory chain in patients with hepatic involvement. Mol Genet Metab 2005;86:462–465.
Tadiboyina VT, Rupar A, Atkison P, et al. Novel mutation in DGUOK in hepatocerebral mitochondrial DNA depletion syndrome associated with cystathioninuria. Am J Med Genet A 2005;135:289–291.
Wang L, Limongelli A, Vila MR, Carrara F, Zeviani M, Eriksson S. Molecular insight into mitochondrial DNA depletion syndrome in two patients with novel mutations in the deoxyguanosine kinase and thymidine kinase 2 genes. Mol Genet Metab 2005;84:75–82.
Freisinger P, Fütterer N, Lankes E, et al. Hepatocerebral mitochondrial DNA depletion syndrome caused by deoxyguanosine kinase (DGUOK) mutations. Arch Neurol 2006;63:1129–1134.
Alberio S, Mineri R, Tiranti V, Zeviani M. Depletion of mtDNA: syndromes and genes. Mitochondrion 2007;7:6–12.
Mousson de Camaret B, Taanman JW, Padet S, et al. Kinetic properties of mutant deoxyguanosine kinase in a case of reversible hepatic mtDNA depletion. Biochem J 2007;402:377–385.
Dimmock DP, Dunn JK, Feigenbaum A, et al. Abnormal neurological features predict poor survival and should preclude liver transplantation in patients with deoxyguanosine kinase deficiency. Liver Transpl 2008;14:1480–1485.
Dimmock DP, Zhang Q, Dionisi-Vici C, et al. Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum Mutat 2008;29:330–331.
Lee NC, Dimmock D, Hwu WL, et al. Simultaneous detection of mitochondrial DNA depletion and single-exon deletion in the deoxyguanosine gene using array-based comparative genomic hybridisation. Arch Dis Child 2009;94:55–58.
Hanchard NA, Shchelochkov OA, Roy A, et al. Deoxyguanosine kinase deficiency presenting as neonatal hemochromatosis. Mol Genet Metab 2011;103:262–267.
Ronchi D, Garone C, Bordoni A, et al. Next-generation sequencing reveals DGUOK mutations in adult patients with mitochondrial DNA multiple deletions. Brain 2012;135:3404–3415.
Mandel H, Hartman C, Berkowitz D, Elpeleg ON, Manov I, Iancu TC. The hepatic mitochondrial DNA depletion syndrome: ultrastructural changes in liver biopsies. Hepatology 2001;34:776–784.
Spinazzola A, Viscomi C, Fernandez-Vizarra E, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet 2006;38:570–575.
Karadimas CL, Vu TH, Holve SA, et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am J Hum Genet 2006;79:544–548.
Wong LJ, Brunetti-Pierri N, Zhang Q, et al. Mutations in the MPV17 gene are responsible for rapidly progressive liver failure in infancy. Hepatology 2007;46:1218–1227.
Navarro-Sastre A, Martín-Hernández E, Campos Y, et al. Lethal hepatopathy and leukodystrophy caused by a novel mutation in MPV17 gene: description of an alternative MPV17 spliced form. Mol Genet Metab 2008;94:234–239.
Spinazzola A, Santer R, Akman OH,et al. Hepatocerebral form of mitochondrial DNA depletion syndrome: novel MPV17 mutations. Arch Neurol 2008;65:1108–1113.
Kaji S, Murayama K, Nagata I, et al. Fluctuating liver functions in siblings with MPV17 mutations and possible improvement associated with dietary and pharmaceutical treatments targeting respiratory chain complex II. Mol Genet Metab 2009;97:292–296.
Parini R, Furlan F, Notarangelo L, et al. Glucose metabolism and diet-based prevention of liver dysfunction in MPV17 mutant patients. J Hepatol 2009;50:215–221.
El-Hattab AW, Li FY, Schmitt E, Zhang S, Craigen WJ, Wong LJ. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: new patients and novel mutations. Mol Genet Metab 2010;99:300–308.
Merkle AN, Nascene DR, McKinney AM. MR imaging findings in the reticular formation in siblings with MPV17-related mitochondrial depletion syndrome. AJNR Am J Neuroradiol 2012;33:E34-35.
El-Hattab AW, Scaglia F, Craigen WJ, Wong LJ. MPV17-related hepatocerebral mitochondrial DNA depletion syndrome. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK92947/. Updated 17 May 2012. Accessed 14 Nov 2012.
Blakely EL, Butterworth A, Hadden RD, et al. MPV17 mutation causes neuropathy and leukoencephalopathy with multiple mtDNA deletions in muscle. Neuromuscul Disord 2012;22:587–591.
Van Goethem G, Dermaut B, Lofgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 2001;28:211–212.
Lamantea E, Tiranti V, Bordoni A, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol 2002;52:211–219.
Van Goethem G, Martin JJ, Dermaut B, et al. Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord 2003;13:133–142.
Filosto M, Mancuso M, Nishigaki Y, et al. Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol 2003;60:1279–1284.
Finsterer J, Zarrouk Mahjoub S. Epilepsy in mitochondrial disorders. Seizure. 2012;21:316–321.
Rahman S. Mitochondrial disease and epilepsy. Dev Med Child Neurol 2012;54:397–406.
Fadic R, Russell JA, Vedanarayanan VV, Lehar M, Kuncl RW, Johns DR. Sensory ataxic neuropathy as the presenting feature of a novel mitochondrial disease. Neurology 1997;49:239–245.
Winterthun S, Ferrari G, He L, et al. Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 2005;64:1204–1208.
Hakonen AH, Heiskanen S, Juvonen V, et al. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet 2005;77:430–441.
Tzoulis C, Engelsen BA, Telstad W, et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain 2006;129:1685–1692.
Worle H, Kohler B, Schlote W, Winkler P, Bastanier CK. Progressive cerebral degeneration of childhood with liver disease (Alpers-Huttenlocher disease) with cytochrome oxidase deficiency presenting with epilepsia partialis continua as the first clinical manifestation. Clin Neuropathol 1998;17:63–68.
Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Karpinski NC, Haas RH. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers syndrome. Ann Neurol 1999;45:54–58.
Gauthier-Villars M, Landrieu P, Cormier-Daire V, et al. Respiratory chain deficiency in Alpers syndrome. Neuropediatrics 2001;32:150–152.
Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol 2004;55:706–712.
Ferrari G, Lamantea E, Donati A, et al. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gammaA. Brain 2005;128:723–731.
Nguyen KV, Østergaard E, Ravn SH, et al. POLG mutations in Alpers syndrome. Neurology 2005;65:1493–1495.
Nguyen KV, Sharief FS, Chan SS, Copeland WC, Naviaux RK. Molecular diagnosis of Alpers syndrome. J Hepatol 2006;45:108–116.
Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase γ gene. Brain 2006;129:1674–1684.
Wong LJ, Naviaux RK, Brunetti-Pierri N, et al. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum Mutat 2008;29:E150-E172.
Milone M, Benarroch EE, Wong LJ. POLG-related disorders: defects of the nuclear and mitochondrial genome interaction. Neurology 2011;77:1847–1852.
Cohen BH, Chinnery PF, Copeland WC. POLG-related disorders. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK26471/. Updated 11 October 2012. Accessed 14 Nov 2012.
Tang S, Dimberg EL, Milone M, Wong LJ. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)-like phenotype: an expanded clinical spectrum of POLG1 mutations. J Neurol 2012;259:862–868.
Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann Neurol 2001;49:377–383.
Koskinen T, Santavuori P, Sainio K, Lappi M, Kallio AK, Pihko H. Infantile onset spinocerebellar ataxia with sensory neuropathy: a new inherited disease. J Neurol Sci 1994;121:50–56.
Koskinen T, Sainio K, Rapola J, Pihko H, Paetau A. Sensory neuropathy in infantile onset spinocerebellar ataxia (IOSCA). Muscle Nerve. 1994;17:509–515.
Koskinen T, Pihko H, Voutilainen R. Primary hypogonadism in females with infantile onset spinocerebellar ataxia. Neuropediatrics 1995;26:263–266.
Koskinen T, Valanne L, Ketonen LM, Pihko H. Infantile-onset spinocerebellar ataxia: MR and CT findings. AJNR Am J Neuroradiol 1995;16:1427–1433.
Hartley JN, Booth FA, Del Bigio MR, Mhanni AA. Novel autosomal recessive c10orf2 mutations causing infantile-onset spinocerebellar ataxia. Case Rep Pediatr 2012;2012:303096.
Kiechl S, Horváth R, Luoma P, et al. Two families with autosomal dominant progressive external ophthalmoplegia. J Neurol Neurosurg Psychiatry 2004;75:1125–1128.
Jeppesen TD, Schwartz M, Colding-Jørgensen E, Krag T, Hauerslev S, Vissing J. Phenotype and clinical course in a family with a new de novo Twinkle gene mutation. Neuromuscul Disord 2008;18:306–309.
Virgilio R, Ronchi D, Hadjigeorgiou GM,et al. Novel Twinkle (PEO1) gene mutations in mendelian progressive external ophthalmoplegia. J Neurol 2008;255:1384–1391.
Sarzi E, Goffart S, Serre V, et al. Twinkle helicase (PEO1) gene mutation causes mitochondrial DNA depletion. Ann Neurol 2007;62:579–587.
Hakonen AH, Isohanni P, Paetau A, Herva R, Suomalainen A, Lönnqvist T. Recessive Twinkle mutations in early onset encephalopathy with mtDNA depletion. Brain 2007;130:3032–3040.
Shoffner JM. Mitochondrial neurogastrointestinal encephalopathy disease. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1179/. Updated 11 May 2010. Accessed 14 Nov 2012.
Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology 1994;44:721–727.
Papadimitriou A, Comi GP, Hadjigeorgiou GM, et al. Partial depletion and multiple deletions of muscle mtDNA in familial MNGIE syndrome. Neurology 1998;51:1086–1092.
Perez-Atayde AR, Fox V, Teitelbaum JE, et al. Mitochondrial neurogastrointestinal encephalomyopathy: diagnosis by rectal biopsy. Am J Surg Pathol 1998;22:1141–1147.
Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999;283:689–692.
Nishino I, Spinazzola A, Papadimitriou A, et al. Mitochondrial neurogastrointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol 2000;47:792–800.
Teitelbaum JE, Berde CB, Nurko S, Buonomo C, Perez-Atayde AR, Fox VL. Diagnosis and management of MNGIE syndrome in children: case report and review of the literature. J Pediatr Gastroenterol Nutr 2002;35:377–383.
Vissing J, Ravn K, Danielsen ER, et al. Multiple mtDNA deletions with features of MNGIE. Neurology 2002;59:926–929.
Nishigaki Y, Martí R, Copeland WC, Hirano M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J Clin Invest 2003;111:1913–1921.
Marti R, Spinazzola A, Tadesse S, Nishino I, Nishigaki Y, Hirano M. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem 2004;50:120–124.
Hirano M, Nishigaki Y, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes. Neurologist 2004;10:8–17.
Blondon H, Polivka M, Joly F, Flourie B, Mikol J, Messing B. Digestive smooth muscle mitochondrial myopathy in patients with mitochondrial-neuro-gastro-intestinal encephalomyopathy (MNGIE). Gastroenterol Clin Biol 2005;29:773–778.
Giordano C, Sebastiani M, Plazzi G, et al. Mitochondrial neurogastrointestinal encephalomyopathy: evidence of mitochondrial DNA depletion in the small intestine. Gastroenterology 2006;130:893–901.
Giordano C, Sebastiani M, De Giorgio R, et al. Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am J Pathol 2008;173:1120–1128.
Finsterer J. Mitochondrial disorders, cognitive impairment and dementia. J Neurol Sci 2009;283:143–148.
Finsterer J. Inherited mitochondrial neuropathies. J Neurol Sci 2011;304:9–16.
Wong LJ. Mitochondrial syndromes with leukoencephalopathies. Semin Neurol 2012;32:55–61.
Szigeti K, Wong LJ, Perng CL, et al. MNGIE with lack of skeletal muscle involvement and a novel TP splice site mutation. J Med Genet 2004;41:125–129.
Bicknese AR, May W, Hickey WF, Dodson WE. Early childhood hepatocerebral degeneration misdiagnosed as valproate hepatotoxicity. Ann Neurol 1992;32:767–775.
Saneto RP, Lee I-C, Koenig MK, et al. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure 2010;19:140–146.
Feranchak AP, Sokol RJ. Medical and nutritional management of cholestasis in infants and children. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 3 ed. New York, NY: Cambridge University Press; 2007: pp. 190–231.
Hasselmann O, Blau N, Ramaekers VT, Quadros EV, Sequeira JM, Weissert M. Cerebral folate deficiency and CNS inflammatory markers in Alpers disease. Mol Genet Metab 2010;99:58–61.
Gold DR, Cohen BH. Treatment of mitochondrial cytopathies. Semin Neurol 2001;21:309–325.
Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007;35:235–242.
Saito K, Kimura N, Oda N, et al. Pyruvate therapy for mitochondrial DNA depletion syndrome. Biochim Biophys Acta 2012;1820:632–636.
Bulst S, Holinski-Feder E, Payne B, et al. In vitro supplementation with deoxynucleoside monophosphates rescues mitochondrial DNA depletion. Mol Genet Metab 2012;107:95–103.
Kelly DA. Liver transplantation: to do or not to do? Pediatr Transplant 2000;4:170–172.
Lara MC, Valentino ML, Torres-Torronteras J, Hirano M, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): biochemical features and therapeutic approaches. Biosci Rep 2007;27:151–163.
Yavuz H, Ozel A, Christensen M, et al. Treatment of mitochondrial neurogastrointestinal encephalomyopathy with dialysis. Arch Neurol 2007;64:435–438.
Lara MC, Weiss B, Illa I, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology 2006;67:1461–1463.
Hirano M, Martí R, Casali C, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology 2006;67:1458–1460.
Rahman S, Hargreaves IP. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology 2007;68:1872.
Halter J, Schüpbach WM, Casali C, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant 2011;46:330–337.
Filosto M, Scarpelli M, Tonin P, et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol 2012;259:2699–2706.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 583 kb)
Rights and permissions
About this article
Cite this article
El-Hattab, A.W., Scaglia, F. Mitochondrial DNA Depletion Syndromes: Review and Updates of Genetic Basis, Manifestations, and Therapeutic Options. Neurotherapeutics 10, 186–198 (2013). https://doi.org/10.1007/s13311-013-0177-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-013-0177-6