Abstract
Therapeutic hypothermia (TH) is the intentional reduction of core body temperature to 32°C to 35°C, and is increasingly applied by intensivists for a variety of acute neurological injuries to achieve neuroprotection and reduction of elevated intracranial pressure. TH improves outcomes in comatose patients after a cardiac arrest with a shockable rhythm, but other off-label applications exist and are likely to increase in the future. This comprehensive review summarizes the physiology and cellular mechanism of action of TH, as well as different means of TH induction and maintenance with potential side effects. Indications of TH are critically reviewed by disease entity, as reported in the most recent literature, and evidence-based recommendations are provided.
Similar content being viewed by others
Introduction
Intensivists are increasingly using therapeutic hypothermia (TH) after acute neurological injures for its neuroprotective effects and ability to reduce elevated intracranial pressure. The 2 sentinel multicenter, randomized, controlled trials, which marked the beginning of a new era of TH, were published in 2002, and these trials established firm evidence for the beneficial use of hypothermia as a therapy to mitigate neurological injury after cardiac arrest [1, 2].
In this review, we will discuss definitions of TH, its physiology and mechanism of action, means of cooling, potential side effects, and the existing evidence and indications of TH (excluding normothermia) in neurologically injured patients.
Defining Therapeutic Hypothermia
TH is defined as intentional reduction of core body temperature (Tc) to 32°C to 35°C [3], although no consensus exists regarding the exact level of cooling. Experts favor the following definitions: mild hypothermia (Tc34°C to 35.9°C), moderate hypothermia (Tc 32°C to 33.9°C), moderate-deep hypothermia (Tc 30°C to 31.9°C), and deep hypothermia (Tc < 30°C) [3]. Despite the lack of consensus in terminology, previous studies demonstrated that body temperature does not need to be less than 32°C to achieve neuroprotection [1, 2, 4–7]. In fact, induced hypothermia to temperatures below 32°C has been associated with significant side effects, such as refractory cardiac arrhythmias and coagulopathies that can be very difficult to manage [8, 9].
Historical Aspects
The use of hypothermia for clinical purposes has ancient roots. Hippocrates advocated the packing of wounded soldiers in snow and ice in 400 BC [10]. Clinical interest in hypothermia was regained in the 1930s and 1940s, with observations and case reports describing successful resuscitation of drowning victims who were hypothermic, even after prolonged periods of asphyxia [11]. The first scientific report describing clinical applications of TH in traumatic brain injury (TBI) was published in 1943 [12]. Subsequently, series of case reports using deep-to-moderate hypothermia (Tc 30°C to 34°C) after cardiac arrest were published in the late 1950s and early 1960s [8, 9, 13]. Due to adverse collateral effects observed at very low temperatures, 30 years passed with no further clinical investigations of hypothermia. Finally, in the 1990s laboratory studies demonstrated the benefit of mild hypothermia in dogs after cardiac arrest [14]. In the last decade, pivotal randomized clinical trials have provided direct evidence of a benefit of mild hypothermia to improve neurological outcome after cardiac arrest and perinatal asphyxia [1, 2, 4–7].
Physiology and Mechanisms of Action
Hypothermia promotes its therapeutic effects in the secondary or latent phase after the initial injury [15]. This phase evolves within hours following the neurological insult and is characterized by the initiation of cascades of acute inflammatory response, vasogenic and cytotoxic edema, impaired brain metabolism, and apoptosis. These lead to delayed neuronal loss, which may be preventable by hypothermia [16].
Detrimental Effects of Fever
Fever (body temperature >38.3°C) occurs in up to 70% of neurologically injured patients, and typically it is not an isolated event, but rather a sustained response seen for as long as 2 weeks following injury [17, 18]. Elevated body temperature can be secondary to infection or a consequence of severe neurological insults. Only half of the febrile episodes are attributable to infection, with nosocomial pulmonary infections representing the largest contributor [19]. Observational and retrospective studies have demonstrated that fever is associated with poor neurological outcome in the first 72 h after cardiac arrest [20, 21], stroke [22], intracerebral hemorrhage [23], subarachnoid hemorrhage [24], and traumatic brain injury [25–27]. In spinal cord injuries, animal studies have revealed that induced hyperthermia immediately after the injury is associated with increased tissue damage and worse outcomes compared to normothermic conditions [28].
Elevated body temperature exacerbates the inflammatory cascade [29–31] and increases neuronal excitoxicity [32]. In an experimental microdialysis study of focal ischemia, glutamate release was significantly higher in hyperthermic than normothermic rats, indicating the importance of focal brain temperature on neurotransmitter release [33].
Pathophysiology
Therapeutic hypothermia is neuroprotective by attenuating secondary injury after the primary neurological insult. The underlying mechanisms involve multiple cellular and molecular pathways. It is clinically effective because of its global effects on all aspects of these pathophysiologic processes. The most obvious physiologic consequence of hypothermia is reduction of cerebral metabolism, as estimated by glucose and oxygen consumption. It has been shown that for every 1°C drop in body temperature, cerebral metabolic rate decreases by 6 to 7% [34, 35]. Energy depletion as in ischemia or abrupt tissue injury, as in TBI, is followed by necrosis and apoptosis. Hypothermia can alter apoptosis by inhibiting caspase activation, decreasing mitochondria dysfunction, and protecting cell wall integrity [36–38]. At a cellular level, interruption of oxygen supply initiates anaerobic glycolysis resulting in metabolic acidosis. The subsequent influx and accumulation of calcium is exacerbated by ion pump failure. This high concentration of calcium leads to release of excitatory neurotransmitter glutamate into the extracellular space. Excess glutamate triggers the excitotoxic cascade that can ultimately result in cell injury and death [39]. Even mild decreases in temperature have been shown to ameliorate these detrimental effects [40–42]. Brain injury activates the inflammatory/immune response [43, 44]. Glial and endothelial cells mediate this by releasing cytokines and interleukins that in turn promote leukocyte diapedesis across the blood brain barrier [45, 46]. This inflammatory cascade causes additional neurotoxicity and apoptosis. There is strong evidence in experimental and clinical studies that hypothermia suppresses multiple aspects of this inflammatory reaction [30, 47, 48]. By the same token, mounting of the inflammatory response increases vascular permeability leading to vasogenic edema [49]. Concurrently, cytotoxic edema also develops via direct free radical damage, homeostatic failure, and the same inflammatory response. These parallel processes accrue to produce brain edema and increased intracranial pressure. Mild hypothermia can reduce blood brain barrier disruption, preserve cell membrane integrity, and thereby decrease intracranial pressure (ICP)-related complications [49, 50].
Damaged brain tissue can have higher temperature than noninjured areas [51–53]. The difference between brain and body temperature can increase in brain-injured patients by 0.1°C to 2°C [54, 55]. The injured area may have a higher temperature secondary to higher cellular metabolism from activation of the previously mentioned mechanisms. The injured tissue cannot dissipate heat secondary to lack of blood flow drainage because of local brain edema [56, 57]. This process can quickly tumble into a vicious cycle, especially in the setting of systemic fever [58]. Hypothermia arrests this deleterious process and allows for dissipation of heat generated by these local destructive processes.
Initiation of Therapeutic Hypothermia
Animal models suggest that any delay in the initiation of TH may diminish or even abrogate the beneficial effect of this therapy. Unfortunately, no data are available from large scale clinical trials to confirm the time dependence of treatment in humans. Nevertheless, it seems reasonable that hypothermia should be initiated as early as possible after the cardiac arrest [59].
TH requires the patients to be on mechanical ventilation with stable hemodynamic support, including intravenous fluids, and, if necessary, inotropic agents or vasopressors [59]. Before TH after cardiac arrest is induced, sedation, analgesia, and paralysis with neuromuscular blocking agents should be initiated to prevent shivering and discomfort. Induction of TH for ICP management may not always require a paralytic agent.
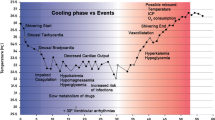
The process of TH is divided into 3 phases: 1) induction, 2) maintenance, and 3) rewarming. During the induction phase, multiple collateral effects are common, which can be minimized by cooling patients as quickly as possible to be able to reach the more hemodynamically stable maintenance phase. This can be accomplished by using a combination of different cooling methods, such as infusion of cold fluids, ice-packs, intranasal cooling, surface cooling devices, or intravascular cooling. Measurement of Tc is essential in target therapeutic management, which should be measured at central sites. For Tc measurements, the esophageal probe is preferred, but may be influenced by the temperature of fluids introduced via the nasogastric tube. The bladder temperature probe has been shown to be reliable in measuring Tc, unless the patient experiences oliguria. Once the Tc decreases below 33.5°C, the patient tends to become more stable, with less risk for fluid loss or intracellular shifts, a cessation or significant diminishment of shivering. During the maintenance phase, tight control of the target temperature should be achieved, with minor or no fluctuations (maximum, 0.2°C to 0.5°C). In this phase, attention should shift toward the prevention of longer term side effects, such as pneumonia, wound infections, and bedsores [60]. Finally, the rewarming phase can lead to severe electrolyte abnormalities (mostly hyperkalemia), caused by shifts from the intracellular to the extracellular compartment, and elevation of ICP. Both can be largely prevented by slow and controlled rewarming, with a warming rate of 0.2°C to 0.5°C/h. This allows the kidneys to excrete the excess potassium and prevents rebound ICP elevations [3, 61]. A detailed description of the physiological changes and potential side effects of hypothermia is listed in Table 1.
For all survivors that have been cooled after cardiac arrest in whom a primary cardiac origin such as an ST-elevation myocardial infarction (STEMI) is suspected, revascularization should be considered [62]. Combining TH with primary percutaneous coronary intervention (PCI) in STEMI is emerging as a new approach to further improve outcomes, and may be more efficacious than either therapy alone. The role of urgent PCI in hypothermic patients after a non-STEMI is uncertain [62].
Determination of the prognosis after cardiac arrest may be difficult in patients undergoing TH. Both human and animal data suggest that monitoring with electroencephalography (EEG) and somatosensory-evoked potentials may correlate with neurological outcomes [63–66]. The bi-spectral index, which is a processed, simplified EEG monitoring tool, quantifies EEG waves and allows monitoring of the level of consciousness. It is commonly used in the operating room to measure the depth of anesthesia, and it ranges from zero (equivalent to a fully suppressed isoelectric EEG) to 100 (awake patient) [67]. Two small cohort studies have suggested that bi-spectral index may predict early neurological outcome after cardiac arrest [68, 69]. In addition, it may be used to confirm the level of sedation during the period of neuromuscular blockade. Additional research is warranted to confirm these findings.
Monitoring of paralysis depth with train-of-four testing is commonly used in nonhypothermic patients. However, caution is warranted during TH because peripheral nerve conduction is significantly slowed by cooling, and train-of-four testing may not be a reliable monitoring method at low body temperatures [70].
Cooling Methods
Cooling can be categorized as systemic or local brain cooling, as well as intravascular versus surface cooling. The method of cooling may be chosen based on specific parameters, such as induction rate to target temperature, stability of temperature control, rate of adverse events, profile of physiologic complications, practicality, and cost in various clinical settings.
Surface Cooling
The general principle of surface cooling relies on the creation of a temperature gradient between the skin and the core. Some surface cooling techniques may require manual temperature adjustments, whereas others have automated feedback control mechanisms. “Conventional surface cooling” typically refers to cooling with ice packs or air-circulating cooling blankets/mattresses. This was the original method used on patients in 1 of the sentinel studies demonstrating improved survival outcomes in cardiac arrest with therapeutic hypothermia [1]. The cooling rate for ice packs is approximately 1°C/h [3], and these are commonly placed around the head, neck, groin, and axillae. The rate for air-circulating cooling blankets is slower at 0.5°C/h [3], and these are placed over or under the patient. Disadvantages to the conventional cooling methods include difficult to control temperatures, a slow cooling rate, frequent need to replace ice packs in the maintenance phase, and difficulties to control rewarming speed. Although conventional cooling methods are more readily accessible and inexpensive, they also have the risk of overcooling, which may lead to coagulopathy, skin damage, and increased shivering. In a retrospective study by Merchant et al. [71] of 32 patients in 3 large centers who underwent surface cooling using a mattress/blanket or ice bag, 63% of patients had temperature less than 32°C for more than 1 h. Temperature fluctuations also occurred throughout both the induction and maintenance phases. This phenomenon has been consistently demonstrated in subsequent studies comparing different cooling methods [72–74]. Fans, washing the patient with alcohol baths, and ice immersions should not be used, as they are unpractical in the intensive care unit setting and ineffective for the maintenance and rewarming phases [3].
More sophisticated surface cooling devices with the ability to cool quickly and reliably and capable of automatic temperature feedback have been invented. The Arctic Sun Temperature Management System (Medivance, Louisville, CO) uses 4 water-circulating pads lined with a hydrogel facilitating heat conduction between the skin and the pads. The pads are placed on the back, abdomen, and both thighs covering approximately 40% of body surface area [75]. The induction rate is about 1.5°C to 2.0°C/h. In the first prospective, randomized trial using this device by Mayer et al. [75] in 47 patients with various neurological injuries, Arctic Sun (Medivance) was compared to water-circulating blankets to achieve normothermia in fever. In this study, the Arctic Sun group experienced a 75% reduction in fever burden compared to the group using water-circulating blankets. Patients also spent less time being febrile and were normothermic faster [75]. In this study, shivering was more common in the Arctic Sun group at 39% of the patients compared to 8% of conventionally treated patients. The prevalence of shivering among different surface cooling techniques is difficult to quantify, given that few studies report shivering rates. In the reported comparison study, the authors did not find a statistically significant difference in mean GCS score between the 2 cooling methods, and it remains unclear whether decreasing fever burden improves outcome. Disadvantages of this gel pad system include high cost, lack of reusability, and the potential for skin breakdown from cutaneous vasoconstriction, especially in patients that already require systemic vasopressors for hemodynamic instability [75]. There are other wrapping type surface cooling techniques available that also use servomechanism temperature control, including the Blanketrol device (Cincinnati Sub-Zero, Cincinnati, OH). These less expensive devices encircle instead of adhering directly to the patient’s skin [3, 76], which decreases efficacy.
Pre-refrigerated cooling pads are highly portable and can be stored in a cooling box inside ambulances. One example is the Emcools Pad System (Emcools, Vienna, Austria), which consists of pre-cooled hydrogel pads. Uray and Malzer [77] studied the feasibility of these pads for out-of-hospital surface cooling in 15 patients, initiated at an average of 12 minutes after return of spontaneous circulation. Target temperature of 33°C was achieved within an average of 50 minutes. All subjects were cooled for 24 h by exclusively using the pads. When the target temperature rose to greater than 33.5°C, additional pads were applied to the thorax and abdomen. Advantages of this pad include ability to initiate hypothermia in the field, portability, and ease of use. A disadvantage to this pad system is the necessity for manual temperature control with a high probability of uncontrolled fluctuations.
Water immersion is the fastest method for lowering core body temperature [3]. The automated cold water immersion Thermosuit System (Life Recovery Systems, Kinnelon, NJ) works by the convective-immersion mechanism and circulates ice water from a perforated top-sheet and an under-blanket across the skin surface at a rate of 14 L/min [78]. In a swine study, it has been shown to achieve target temperature of 33°C in 9 minutes. In humans, it had a median cooling time of 3°C/h with median time-to-target time of 37 minutes. Once target temperature is reached, the water is purged and the patient is removed from the device. Disadvantages of such fast cooling rates include increased risks of electrolyte shifts, arrhythmias, and the potential for overcooling. In addition, it is unclear whether this system should be used only as an induction method in addition to other modalities for maintaining steady temperature control.
Intravascular Cooling
Infusion of Ice-Cold Fluids
One major disadvantage of surface cooling is the slow speed of cooling. Intravascular cooling bypasses the peripheral compartment and can achieve cooling of the core in a smaller amount of time. The use of intravenous ice-cold fluids in patients resuscitated from cardiac arrest as a rapid, safe, and easy means of inducing hypothermia is supported by several studies [79–81]. One study used 4°C Lactated Ringer’s solution infused for 30 min in 22 resuscitated cardiac arrest patients and showed a decrease in median Tc of 1.7°C, as well as beneficial hemodynamic, renal, and acid–base effects [80]. Infusing up to 30 ml/kg ice-cold 4°C saline has been shown to effectively and quickly lower Tc by 4.0 ± 0.3°C within 60 minutes when used in combination with surface cooling [79]. The same study abated concerns that intravenous cooling may lead to hypotension and heart failure. There was an increase in mean arterial pressure by 15 mmHg, and no patients developed pulmonary edema [79].
Intravascular Cooling Catheters
In addition to the infusion of ice-cold fluids, catheter-based cooling systems exist. Currently, there are 2 commercially available devices with similar designs (the Alsius System, Alsius Corp., Irvine, CA and the Celsius Control System, Innercool Therapies, San Diego, CA). Both function as a central venous catheter with an additional cooling system. The Alsius (Alsius Corp.) catheter has 3 cylindrical balloons filled with saline in a closed system near the tip of the catheter with a feedback system to the bedside unit. Using a core temperature monitor, the patient’s temperature is regulated via an automatic adjustment of the saline temperature in the cylindrical balloons [82]. The Celsius (Innercool Therapies) catheter has a flexible distal metallic heat transfer unit, which contains cold saline and a patient body temperature sensing probe. It is also connected to a bedside unit that automatically regulates the circulation temperature to maintain core temperature [83]. In both systems, catheters are inserted into a central vein, most commonly the inferior vena cava via femoral access.
Advantages of endovascular cooling devices include shorter time to target temperature and the ability to maintain a more stable temperature compared to conventional methods, such as ice packs and cooling mattresses [84, 85]. The disadvantages of endovascular cooling devices include that it is an invasive procedure and it carries a high risk of deep venous thrombosis (DVT), bacteremia, and sepsis. In a study of TBI patients, the incidence of asymptomatic DVT was as great as 50% when using endovascular cooling devices [86]. A sixfold increase of the nosocomial bloodstream infection rate (13% vs a control rate of 2%) was found in another study of endovascular cooling catheters [82]. Therefore, endovascular cooling is currently only used in patients in whom cooling is expected to last no longer than 24 h, with prompt removal of the catheter after this time period to prevent DVT and bacteremia.
Head-to-head comparisons of surface and endovascular cooling devices after cardiac arrest have revealed similar cooling efficacy and numbers of adverse effects, except that the surface group experienced more hyperglycemia, whereas the endovascular group experienced more hypomagnesemia [87]. Although it has been proposed that catheter cooling may result in less shivering [75], this study did not show any difference in the rate of shivering between the 2 methods [87]. The study was not sufficiently powered to find a difference in survival or neurological outcome.
Intranasal Cooling
Recently, brain cooling via local methods has been described as a rapid, portable, and less invasive means of hypothermia induction. Two systems exist: 1) an evaporative system using a coolant sprayed into the nose, and 2) a conductive system that uses cold saline circulated via intranasal balloons. In the first method, nasal catheters connected to a bottle containing perfluorochemicals are inserted into the nostrils. The coolant with high evaporative properties is sprayed along the nasal passage ways. In the human body, these chemicals are biologically nonreactive [88]. This method was evaluated in a safety and feasibility study of 84 cardiac arrest patients in the emergency room setting [88]. Patients were solely cooled with this device for 1 h and were then transitioned to other systemic methods. The study showed that nasopharyngeal cooling reduced tympanic temperature by median of 2.3°C and core temperature by 1.1°C. This method was also studied as an adjunct to systemic cooling in a multicenter study of 200 cardiac arrest patients in the pre-hospital setting during ongoing resuscitation [89]. Time-to-target temperature of 34°C was shorter in the treatment group with intranasal cooling started in the field [89]. In a recent study using intranasal cooling in a more heterogeneous cohort of 15 patients in the intensive care unit with intracerebral hemorrhage, trauma, and ischemic stroke, patients underwent induction of cooling with the RhinoChill System (BeneChillI, San Diego, CA) [91]. Core, brain, and tympanic temperatures were reduced by 1.1°C, 1.4°C, and 2.2°C, respectively, within 60 minutes of the induction phase. In all studies, the most frequent device-related adverse events were nasal discoloration and epistaxis. In a recent study of ten healthy volunteers, Covaciu et al. [90] demonstrated that brain cooling using intranasal cooling with circulating cold saline (20°C) balloon catheters can be achieved affectively in non-intubated patients without shivering. The indication to cool awake patients remains to be established. One disadvantage of intranasal cooling devices is the exclusion of patients with basal skull fractures. Intranasal cooling has not been studied for the maintenance of hypothermia.
Cool Caps for Infants
Selective head cooling with mild systemic hypothermia in infants suffering from hypoxic-ischemic encephalopathy (HIE) as a result of perinatal asphyxia was first piloted in a randomized controlled trial in 1998 [91]. Using a cooling cap in neonates is considered more efficient compared to adults because of large head-to-torso size ratio. Reduction in temperature is achieved by running 10°C water through a coil of tubing wrapped around the infant’s scalp for 72 hours [91]. Other studies have showed improvement in short-term computed tomographic changes [92], neurodevelopment outcome at 18 months [5], or combined outcome of severe disability and death [93]. As with other surface cooling methods, some devices are adjusted manually or automatically via a feedback mechanism [93]. Potential adverse events include scalp edema, thrombocytopenia, hypotension, and bradycardia [94]. Recently, the cool cap device was retrospectively compared to a whole body wrap with automatic feedback mechanisms [95]. The cool cap was inferior in maintaining target rectal temperature (76% of the time in cool cap compared to 97% in whole body wrap) and had an overshoot of the temperature by 0.8°C [95].
To date, there have been no prospective clinical trials in children that compare whole body cooling versus selective head cooling with mild systemic hypothermia in terms of mortality and long-term survival characteristics.
Local Cerebral Cooling in Adults
Disadvantages of systemic whole body cooling include shivering and the potential for infections. Similar to the cool cap in infants, local cerebral cooling using a cooling helmet, sometimes in combination with neck cooling, has been tried in the adult [96–99]. However, due to a smaller head-to-body ratio in adults compared to infants, cooling helmets are less commonly used in adults, as the induction rate is slow and maintaining target temperature is difficult [100]. Recently, a different method of local cerebral cooling to achieve normothermia has been studied in a small cohort of febrile subarachnoid hemorrhage patients [101], using 3 differently shaped neck pads consisting of the same hydrogel-coated water circulating pads used by the Arctic Sun System (Medivance, Louisville, CO) covering the carotid triangles. With this method, brain temperature was decreased by 0.5 ± 0.3°C after 5 h. No hemodynamic side effects or shivering was observed. The study did not target hypothermia; therefore, it is not clear how effective this method may be at lower temperature goals.
Evidence-Based Review of the Clinical Application of Therapeutic Hypothermia
Patients in Whom Therapeutic Hypothermia has Been Proven Beneficial
Witnessed Cardiac Arrest with a Shockable Rhythm (Ventricular Fibrillation or Pulseless Ventricular Tachycardia)
The publication of 2 sentinel multicenter, randomized controlled trials in The New England Journal of Medicine in 2002 established firm evidence for the beneficial use of TH in resuscitated patients after cardiac arrest with a shockable rhythm [1, 2].
One of the trials was conducted by the Hypothermia After Cardiac Arrest Study group, and randomized 275 comatose survivors of a cardiac arrest with a shockable rhythm (ventricular fibrillation [VF] or pulseless ventricular tachycardia) to either hypothermia (target temperature 32°C to 34°C, for 24 h with the use of air-cooled blankets) or to standard treatment [1]. At 6-months post-arrest, 55% of the cooled patients had a good outcome compared with 39% of the normothermia group (risk ratio for a favorable outcome with hypothermia, 1.40; 95% confidence interval [CI], 1.08 to 1.81). In the other published trial from Australia, investigators enrolled 77 comatose survivors of a cardiac arrest with an initial shockable rhythm (VF or ventricular tachycardia) [2]. Patients were randomly assigned to hypothermia (target temperature, 33°C for 12 h with the use of ice packs only, initiated in the field) or standard treatment. At the time of hospital discharge, 49% of patients who were cooled had good neurological outcomes compared with 26% of the control group (odds ratio [OR] for a favorable neurological recovery with hypothermia therapy, 5.25; 95% CI, 1.47 to 18.76). A subsequent meta-analysis indicated that the number needed to treat to provide a favorable neurological outcome was 6 [102].
Both trials have received significant criticism, given that neither study included data reflecting the depth or severity of coma before randomization, or took into consideration the arrest and resuscitation times. Furthermore, in the hypothermia groups of both trials, goal temperatures were achieved late after 8 h or more, or not at all. Yet, the results were striking and likely underestimate the beneficial therapeutic effects. As a result, therapeutic hypothermia (cooling to 32-34°C for 12–24 h, see Table 2) is now included as a recommended treatment in the American Heart Association Resuscitation Guidelines of comatose survivors after cardiac arrest with a shockable rhythm [103].
Perinatal Asphyxia
Among term infants, HIE due to acute perinatal asphyxia remains an important cause of neurodevelopmental deficits in childhood. Until the last decade, management of a newborn with HIE had consisted largely of supportive care. In the last 6 years, 3 large, randomized, placebo-controlled trials have shown that TH initiated within 6 h of birth reduces death and disability in these infants [5–7].
The Cool-Cap trial used selective head cooling with mild systemic hypothermia for treatment of asphyxia and enrolled 234 infants [5]. Infants with moderate-to-severe asphyxia confirmed by biochemical, clinical, neurological, and EEG data were included. Patients were cooled to 34 to 35°C for 72 h within 5.5 h of their birth. At the 18-month follow-up, the overall rate of adverse outcome (death or severe disability) was not different between the cooled and the normothermic group (55% vs 66%; Relative risk (RR), 0.61 95% CI, 0.34 to 1.09; p = 0.1). However, after controlling for baseline clinical severity in a post-hoc analysis, hypothermia was shown to improve outcome (OR, 0.52; 95% CI, 0.28 to 0.97).
In a different multicenter trial of 208 patients with perinatal asphyxia [6], infants with HIE were cooled with cooling blankets to 33.5°C within 6 h of birth for 72 h. At the 18-month follow-up, death, or moderate or severe disability was significantly less in the hypothermia group compared to the control group (44% vs 62%; risk ratio, 0.72; 95% CI, 0.54 to 0.95; p = 0.01), without an increase in major disability among survivors.
The Total Body Hypothermia for Neonatal Encephalopathy (TOBY) trial enrolled 325 infants with moderate-to-severe asphyxia [7]. The hypothermia group was cooled to 33°C to 34°C for 72 h using gel packs. At the 18-month follow up, infants in the cooled group had an increased rate of survival without neurological abnormality (relative risk, 1.57; 95% CI, 1.16 to 2.12; p = 0.03). Among survivors, cooling resulted in reduced risks of cerebral palsy (relative risk, 0.67; 95% CI, 0.47 to 0.96; p = 0.03). A recent meta-analysis including 10 randomized controlled trials (n = 1320) revealed the number needed to treat to achieve increased survival with normal neurological function after HIE to be 8 [104].
Although there are no randomized trials in the pediatric population on the effect of therapeutic hypothermia after cardiac arrest, the findings in the adult population prompted the American Heart Association to extend the recommendation of therapeutic hypothermia to young children and adolescents who remain comatose after resuscitation from sudden, witnessed, out-of-hospital VF cardiac arrest (see Table 2) [105].
Patients in Whom Therapeutic Hypothermia has Not Been Proven Beneficial and May be Detrimental
TBI
Several studies have been published, both in children and in adults, during the last 2 decades that have evaluated the effect of hypothermia on outcome following severe TBI (GCS ≤ 8). More than 10 randomized controlled trials have not yielded consistent findings at the effects of therapeutic hypothermia in TBI [106, 107]. A recent Cochrane meta-analysis evaluated the effect of hypothermia to <35°C for at least 12 consecutive hours on outcome in TBI in a total of 23 trials and 1614 patients, and concluded that there was no beneficial effect of hypothermia [108]. One might argue that a delayed initiation of hypothermia after injury might be contributory to the negative findings. However, as has been shown most recently, even the application of very early hypothermia after severe TBI (within 2.5 h after injury) did not confirm the usefulness of hypothermia as a primary neuroprotective treatment [109].
In children, 2 randomized trials could not demonstrate any beneficial effect of hypothermia on outcome [110, 111]. Recently, a large, multicenter, randomized, placebo-controlled trial involving 225 children with severe TBI (GCS ≤ 8) did not reveal a difference between groups, with a tendency toward worse outcome in the hypothermia group (mean temperature, 33.1°C ± 1.2°C) due to a higher mortality rate in patients >7 years of age [112]. A current ongoing trial enrolling children <16 years of age is attempting to show benefit by extending the cooling period (32°C to 33°C) to 48 h with a slow re-warming phase of 1°C every 12 to 24 h [113].
Currently, there is no indication for the use of TH after TBI for neuroprotection. TH, however, may still be useful in TBI in the control of refractory intracranial hypertension.
Patients in Whom the Benefit from Therapeutic Hypothermia is Unclear
Traumatic Intracranial Hypertension
Many clinical trials studying TH in severe TBI reported the effect of hypothermia on intracranial hypertension and found it to be efficacious in reducing elevated ICP [114]. However, the failure of TH on overall outcome after TBI may be due to possible intracranial rebound hypertension associated with rewarming, which can be difficult to control.
In a recent review, TH was compared to other therapies for reduction of elevated ICP [115]. The effect of TH on lowering ICP was as effective as hyperventilation, mannitol, and barbiturates, but less effective than hypertonic saline, cerebrospinal fluid drainage, and decompressive craniectomy.
It has been suggested that TH should be used until ICP is controlled regardless of the length of treatment. This was supported by a Chinese trial of 215 patients with severe TBI, which compared TH for ICP control for 2 days versus 5 days [116]. Cooling began 3 h after injury, and the target temperature was 33°C to 35°C. In the short duration TH group, ICP was observed to rebound significantly with rewarming, although this was not observed in the long duration TH group. The 6-month outcomes were also significantly better in the long duration TH group, with no difference in side effects between groups.
TH may be considered for TBI patients with elevated ICP, keeping in mind that it has not shown to improve long-term neurological outcome after TBI. Also, the optimal target temperature and duration of treatment are unknown (see Table 2).
Ischemic Stroke
TH is a plausible application after acute ischemic stroke, given the beneficial effect after cardiac arrest [1, 2]. A meta-analysis of animal studies including 3353 animals treated with hypothermia after acute stroke indicated a robust benefit of hypothermia in infarct size or functional outcome. Hypothermia reduced infarct size by 44% (95% CI, 40–47%) [117]. However, hypothermia was induced extremely early (prior to the stroke, or within less than 3 h) in these studies, which may not be feasible in clinical practice.
Several small human trials have investigated the safety and feasibility of TH after thrombolysis after ischemic stroke, given safety concerns of increased coagulopathy in TH [118–122]. Currently, a larger randomized, multicenter phase 2/3 trial is being conducted to further evaluate safety and efficacy of TH in ischemic stroke (Clinicaltrials.gov. No. NCT01123161).
Based on the current evidence, it remains unclear whether hypothermia after ischemic stroke is beneficial for improving clinical outcome.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) can be complicated by elevated intracranial pressure, global and focal brain edema, as well as delayed ischemia from cerebral vasospasm. Fever has been associated with worse outcomes [123, 124], so that the most recently published guidelines for SAH recommend aggressive treatment of fever to normothermia [125]. No clear evidence exists in using hypothermia after SAH. A single trial of hypothermia after SAH conducted in Switzerland reported the feasibility and safety of long-term hypothermia (>72 h) in the treatment of severe brain edema after SAH in patients with a Hunt and Hess grade of 4 or 5 [126]. Among 156 patients with SAH, 21 patients were treated with mild hypothermia (target temperature, 33°C to 34°C) combined with barbiturate coma. Nine patients were treated for <72 h and 12 for >72 h. Functional independence at 3 months (Glasgow Outcome Scale score, 4 or 5) did not differ between the 2 groups, as the study was underpowered. In all patients, the most common form of complication was infection. Case reports in patients with SAH stated that rewarming following TH and the side effects from TH may aggravate the pre-existing hypoperfusion [127–130], whereas others claim an uneventful course if rewarming is performed slowly [131]. To date, no evidence exists confirming or refuting the benefit of TH after SAH.
Spinal Cord Injuries
Hypothermia as an acute treatment for traumatic spinal cord injury received popular attention with the 2007 case of a United States professional football player. He had sustained a C3-C4 fracture dislocation with an American Spinal Injury Association scale (ASIA) A during a game. Hypothermia was initiated in the field and continued en route to the hospital, followed by 24 h of systemic cooling. He had almost a complete neurological recovery [132, 133].
Recently, a retrospective study compared 14 patients with complete cervical spinal cord injuries (ASIA A) treated with intravascular hypothermia (target temperature, 33°C) for 48 h to a control group of 14 patients treated without hypothermia [134]. The authors reported that 44% of the hypothermia group versus 21% of the control group had at least a 1-grade improvement in ASIA score. The study lacked sufficient power and no definite differences in outcome were seen. Group differences in timing of surgery and steroids given further complicate the analysis. Until the results of further trials are available, the American Association of Neurological Surgeons and the Congress of Neurological Surgeons agree there is insufficient scientific evidence to support or oppose systemic or local hypothermia for traumatic spinal cord injury [114].
Hepatic Encephalopathy
Hepatic encephalopathy and brain edema leading to intracranial hypertension are 2 major complications in patients with acute liver failure [135]. It is estimated that 20% of patients with acute liver failure die from increased ICP while awaiting transplantation. Given the ability of TH to reduce elevated ICP in TBI, TH has been applied to comatose liver failure patients with brain edema in an attempt to prevent ICP related brain injury while awaiting liver transplantation [114]. In a small observational study of patients with acute liver failure awaiting orthotopic liver transplantation, refractory increased ICP were cooled to a core temperature of 32°C to 33°C for 10 to 118 h, with a median of 32 h [136]. On average, ICP was reduced from 36.5 to 16.3 mmHg at 4 h, which was sustained at 24 h (16.8 ± 1.5 mmHg; p < 0.0001). Thirteen of these patients were then able to undergo successful liver transplant and had complete neurological recovery. Hypothermia was also associated with a significant reduction in arterial ammonia concentration.
In 2007, the United States Acute Liver Failure Study Group published consensus recommendations that “ICP should be monitored for all patients with advanced hepatic encephalopathy who are awaiting orthotopic liver transplantation.” The group also observed that “the induction of moderate hypothermia appears to be promising as a bridge to orthotopic liver transplantation,” but did not make specific recommendations for its use [137].
Cardiac Arrest with Non-Shockable Rhythms
In the last decade, only a few studies have evaluated the potential benefit of induced hypothermia in out-of-hospital cardiac arrest with a non-shockable rhythm, and these have provided conflicting results. In a small randomized placebo-controlled trial of 30 patients, induced hypothermia demonstrated a beneficial effect in non-shockable rhythm patients [96], whereas in a more recent trial, induced hypothermia was related to a trend toward a harmful effect [81]. In observational studies, mixed results were also reported [4].
Recently, a large observational study from France showed no benefit from induced hypothermia in patients presenting with a non-shockable rhythm [4]. Prospectively collected data from 1145 cardiac arrest survivors with shockable and non-shockable rhythms were retrospectively analyzed. Therapeutic hypothermia (target temperature, 32°C to 34°C, cooling for 24 h) confirmed improved outcome in patients with a shockable rhythm (44% vs 29%; adjusted OR, 1.9; 95% CI, 1.18 to 3.06; p < 0.001). In contrast, in the patients who presented with a non-shockable rhythm pulseless electrical activity (PEA/asystole), therapeutic hypothermia did not improve outcome (15% vs 17%, adjusted OR, 0.71; 95% CI, 0.37 to 1.36; p = 0.48) with a trend toward a worse outcome. These results emphasize that benefits of therapeutic hypothermia in patients with shockable rhythms cannot be extrapolated to patients with non-shockable rhythms, calling for a large, randomized, controlled trial of hypothermia in asystole and PEA cardiac arrest.
Conclusions
Therapeutic hypothermia should be applied to inpatients who are unable to follow verbal commands after a cardiac arrest with a shockable rhythm, including children and adults. Therapeutic hypothermia should also be considered in the treatment of moderate-to-severe hypoxic encephalopathy in asphyxiated newborns. Hypothermia should be initiated as soon as possible in these patients to achieve its optimal beneficial effect. Presently, there is no evidence that exists to support the use of hypothermia in patients with acute TBI.
There is considerable potential for hypothermia to have favorable impact on the outcome of elevated intracranial pressure, ischemic stroke, subarachnoid hemorrhage, spinal cord injuries, and hepatic encephalopathy, but further studies are needed to establish its efficacy in improving outcomes in these neurological insults.
The management of side effects seems to be crucial throughout the entire therapeutic process. As intensivists gain more experience with technological advances and the management of complications associated with hypothermia, widespread applications may become more feasible.
References
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med [Clinical Trial Comparative Study Multicenter Study Randomized Controlled Trial] 2002;346:549–556.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med [Clinical Trial Comparative Study Randomized Controlled Trial] 2002;346:557–563.
Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009;37:1101–1120.
Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients? Insights from a large registry. Circulation [Research Support, Non-U.S. Gov't] 2011;123:877–886.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–670.
Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–1584.
Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–1358.
Benson DW, Williams GR Jr., Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959;38:423–428.
Williams GR Jr., Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958;148:462–468.
Hippocrates. De Vetere Medicina. Hippocrates Loeb Classical Library. 460–375 BC; Translation: Jones WHS, Withington ET.
Polderman KH. Application of therapeutic hypothermia in the ICU: opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med 2004;30:556–575.
Fay T. Observations on generalized refrigeration in cases of severe cerebral trauma. Assoc Res Nerv Ment Dis Proc 1943;24:611–619.
Ravitch MM, Lane R, Safar P, Steichen FM, Knowles P. Lightning stroke. Report of a case with recovery after cardiac massage and prolonged artificial respiration. N Engl J Med 1961;264:36–38.
Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke 1992;23:1454–1462.
Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–169.
Drury PP, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Semin Fetal Neonatal Med 2010;15:287–292.
Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med 2004;32:1489–1495.
Kilpatrick MM, Lowry DW, Firlik AD, Yonas H, Marion DW. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery 2000;47:850–856.
Georgilis K, Plomaritoglou A, Dafni U, Bassiakos Y, Vemmos K. Aetiology of fever in patients with acute stroke. J Intern Med 1999;246:203–209.
Takasu A, Saitoh D, Kaneko N, Sakamoto T, Okada Y. Hyperthermia: is it an ominous sign after cardiac arrest? Resuscitation 2001;49:273–277.
Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 2001;161:2007–2012.
Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome : a meta-analysis of studies in patients. Stroke 2000;31:410–414.
Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000;54:354–361.
Fernandez A, Schmidt JM, Claassen J, et al. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology 2007;68:1013–1019.
Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19:869–874.
Stocchetti N, Rossi S, Zanier ER, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med 2002;28:1555–1562.
Jones PA, Andrews PJ, Midgley S, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 1994;6:4–14.
Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res 2007;162:201–217.
Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery 2002;51:195–203.
Dietrich WD, Chatzipanteli K, Vitarbo E, Wada K, Kinoshita K. The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir Suppl 2004;89:69–74.
Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Neurosurgery 2004;55:416–425.
Suehiro E, Fujisawa H, Ito H, Ishikawa T, Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. J Neurotrauma [Research Support, Non-U.S. Gov't] 1999;16:285–297.
Takagi K, Ginsberg MD, Globus MY, Martinez E, Busto R. Effect of hyperthermia on glutamate release in ischemic penumbra after middle cerebral artery occlusion in rats. Am J Physiol 1994;267(5 Pt 2):H1770-H1776.
Ehrlich MP, McCullough JN, Zhang N, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73:191–197.
Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 2003;23:513–530.
Povlishock JT, Buki A, Koiziumi H, Stone J, Okonkwo DO. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir Suppl 1999;73:15–20.
Adachi M, Sohma O, Tsuneishi S, Takada S, Nakamura H. Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res 2001;50:590–595.
Leker RR, Constantini S. Experimental models in focal cerebral ischemia: are we there yet? Acta Neurochir Suppl 2002;83:55–59.
Siesjo BK, Bengtsson F, Grampp W, Theander S. Calcium, excitotoxins, and neuronal death in the brain. Ann N Y Acad Sci 1989;568:234–251.
Globus MY, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem 1988;51:1455–1464.
Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995;65:1704–1711.
Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 1989;20:904–910.
Small DL, Morley P, Buchan AM. Biology of ischemic cerebral cell death. Prog Cardiovasc Dis 1999;42:185–207.
Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev 2005;48:388–399.
Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci 1999;22:504–512.
Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 2002;8:101–105.
Aibiki M, Maekawa S, Ogura S, Kinoshita Y, Kawai N, Yokono S. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma 1999;16:225–232.
Kimura A, Sakurada S, Ohkuni H, Todome Y, Kurata K. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit Care Med 2002;30:1499–1502.
Smith SL, Hall ED. Mild pre- and posttraumatic hypothermia attenuates blood–brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma 1996;13:1–9.
Jurkovich GJ, Pitt RM, Curreri PW, Granger DN. Hypothermia prevents increased capillary permeability following ischemia-reperfusion injury. J Surg Res 1988;44:514–521.
Henker A, Schindler I, Renz A, Beck E. Protein heterogeneity of spinach pullulanase results from the coexistence of interconvertible isomeric forms of the monomeric enzyme. Biochem J 1998;331(Pt 3):929–935.
Verlooy J, Heytens L, Veeckmans G, Selosse P. Intracerebral temperature monitoring in severely head injured patients. Acta Neurochir (Wien) 1995;134:76–78.
Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology 1997;48:762–767.
Henker RA, Brown SD, Marion DW. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery 1998;42:1071–1075.
Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med 1998;26:562–567.
Hayashi N, Hirayama T, Udagawa A, Daimon W, Ohata M. Systemic management of cerebral edema based on a new concept in severe head injury patients. Acta Neurochir Suppl (Wien) 1994;60:541–543.
Piepgras A, Elste V, Frietsch T, Schmiedek P, Reith W, Schilling L. Effect of moderate hypothermia on experimental severe subarachnoid hemorrhage, as evaluated by apparent diffusion coefficient changes. Neurosurgery 2001;48:1128–1135.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37(7 suppl):S186-S202.
Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010;363:1256–1264.
Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality — Part 2: Practical aspects and side effects. Intensive Care Med 2004;30:757–769.
Polderman KH, Callaghan J. Equipment review: cooling catheters to induce therapeutic hypothermia? Crit Care 2006;10:234.
Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation 2011;123:1428–1435.
Rundgren M, Rosen I, Friberg H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive Care Med 2006;32:836–842.
Tiainen M, Kovala TT, Takkunen OS, Roine RO. Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med 2005;33:1736–1740.
Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 2006;66:62–68.
Zandbergen EG, Koelman JH, de Haan RJ, Hijdra A. SSEPs and prognosis in postanoxic coma: only short or also long latency responses? Neurology 2006;67:583–586.
Rosow C, Manberg PJ. Bispectral index monitoring. Anesthesiol Clin North America 2001;19:947–966.
Leary M, Fried DA, Gaieski DF, et al. Neurologic prognostication and bispectral index monitoring after resuscitation from cardiac arrest. Resuscitation 2010;81:1133–1137.
Seder DB, Fraser GL, Robbins T, Libby L, Riker RR. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med 2010;36:281–288.
Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandin B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg 2010;110:1328–1335.
Merchant RM, Abella BS, Peberdy MA, et al. Therapeutic hypothermia after cardiac arrest: unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med 2006;34(12 suppl):S490-S494.
Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation 2010;81:1117–1122.
Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010;81:9–14.
Larsson IM, Wallin E, Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation 2010;81:15–19.
Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–2515.
Seder DB, Van der Kloot TE. Methods of cooling: practical aspects of therapeutic temperature management. Crit Care Med 2009;37(7 suppl):S211-S222.
Uray T, Malzer R. Out-of-hospital surface cooling to induce mild hypothermia in human cardiac arrest: a feasibility trial. Resuscitation 2008;77:331–338.
Howes D, Ohley W, Dorian P, et al. Rapid induction of therapeutic hypothermia using convective-immersion surface cooling: safety, efficacy and outcomes. Resuscitation 2010;81:388–392.
Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005;33:2744–2751.
Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003;56:9–13.
Kim F, Olsufka M, Longstreth WT Jr., et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007;115:3064–3070.
Pichon N, Amiel JB, Francois B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007;11:R71.
Badjatia N, O'Donnell J, Baker JR, et al. Achieving normothermia in patients with febrile subarachnoid hemorrhage: feasibility and safety of a novel intravascular cooling catheter. Neurocrit Care 2004;1:145–156.
Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care 2007;11:R91.
Flint AC, Hemphill JC, Bonovich DC. Therapeutic hypothermia after cardiac arrest: performance characteristics and safety of surface cooling with or without endovascular cooling. Neurocrit Care 2007;7:109–118.
Simosa HF, Petersen DJ, Agarwal SK, Burke PA, Hirsch EF. Increased risk of deep venous thrombosis with endovascular cooling in patients with traumatic head injury. Am Surg 2007;73:461–464.
Tomte O, Draegni T, Mangschau A, Jacobsen D, Auestad B, Sunde K. A comparison of intravascular and surface cooling techniques in comatose cardiac arrest survivors. Crit Care Med 2011;39:443–449.
Busch HJ, Eichwede F, Fodisch M, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation 2010;81:943–949.
Castren M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010;122:729–736.
Covaciu L, Weis J, Bengtsson C, Allers M, Lunderquist A, Ahlstrom H, Rubertsson S. Brain temperature in volunteers subjected to intranasal cooling. Intensive Care Med 2011;37:1277–84
Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102(4 Pt 1):885–892.
Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J Perinatol 2006;26:180–184.
Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157:367–373.
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007(4):CD003311.
Hoque N, Chakkarapani E, Liu X, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics 2010;126:e124-e130.
Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001;51:275–281.
Wang H, Olivero W, Lanzino G, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. J Neurosurg 2004;100:272–277.
Storm C, Schefold JC, Kerner T, et al. Prehospital cooling with hypothermia caps (PreCoCa): a feasibility study. Clin Res Cardiol 2008;97:768–772.
Qiu W, Shen H, Zhang Y, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci 2006;13:995–1000.
Holzer M. Devices for rapid induction of hypothermia. Eur J Anaesthesiol Suppl 2008;42:31–38.
Mink S, Schwarz U, Mudra R, Gugl C, Frohlich J, Keller E. Treatment of resistant Fever: new method of local cerebral cooling. Neurocrit Care 2011;15:107–112.
Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414–418.
Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 suppl 3):S768-S786.
Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 suppl 3):S876-S908.
Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 2001;344:556–563.
Shiozaki T, Hayakata T, Taneda M, et al. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg 2001;94:50–54.
Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev 2009(2):CD001048.
Clifton GL, Valadka A, Zygun D, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol 2011;10:131–139.
Biswas AK, Bruce DA, Sklar FH, Bokovoy JL, Sommerauer JF. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med 2002;30:2742–2751.
Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 2005;56:740–754.
Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008;358:2447–2456.
Adelson PD. Hypothermia following pediatric traumatic brain injury. J Neurotrauma 2009;26:429–436.
Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma 2009;26:455–467.
Schreckinger M, Marion DW. Contemporary management of traumatic intracranial hypertension: is there a role for therapeutic hypothermia? Neurocrit Care 2009;11:427–436.
Jiang JY, Xu W, Li WP, et al. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab 2006;26:771–776.
van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007;130(Pt 12):3063–3074.
Groysman LI, Emanuel BA, Kim-Tenser MA, Sung GY, Mack WJ. Therapeutic hypothermia in acute ischemic stroke. Neurosurg Focus 2011;30:E17.
Krieger DW, De Georgia MA, Abou-Chebl A, et al. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke 2001;32:1847–1854.
De Georgia MA, Krieger DW, Abou-Chebl A, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology 2004;63:312–317.
Lyden PD, Allgren RL, Ng K, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis 2005;14:107–114.d
Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke 2010;41:2265–2270.
Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 2006;34:617–624.
Oliveira-Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology 2001;56:1299–1304.
Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care 2011 Sep;15(2):211–40.
Gasser S, Khan N, Yonekawa Y, Imhof HG, Keller E. Long-term hypothermia in patients with severe brain edema after poor-grade subarachnoid hemorrhage: feasibility and intensive care complications. J Neurosurg Anesthesiol 2003;15:240–248.
Sagher O, Huang DL, Webb RC. Induction of hypercontractility in human cerebral arteries by rewarming following hypothermia: a possible role for tyrosine kinase. J Neurosurg 1997;87:431–435.
Jimbo H, Dohi K, Nakamura Y, et al. Fatal severe vasospasm due to rewarming following hypothermia — case report. Neurol Med Chir (Tokyo) 2000;40:463–466.
Kassell NF, Peerless SJ, Drake CG, Boarini DJ, Adams HP. Treatment of ischemic deficits from cerebral vasospasm with high dose barbiturate therapy. Neurosurgery 1980;7:593–597.
van der Linden J, Ekroth R, Lincoln C, Pugsley W, Scallan M, Tyden H. Is cerebral blood flow/metabolic mismatch during rewarming a risk factor after profound hypothermic procedures in small children? Eur J Cardiothorac Surg 1989;3:209–215.
Yasui N, Kawamura S, Suzuki A, Hadeishi H, Hatazawa J. Role of hypothermia in the management of severe cases of subarachnoid hemorrhage. Acta Neurochir Suppl 2002;82:93–98.
Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine (Phila Pa 1976) 2010;5:E57-E62.
Beauchamp KM, Flierl MA, Stahel PF, et al. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine 2010;35:E57-62. Spine (Phila Pa 1976) 2010;35:1827–1828.
Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery 2010;66:670–677.
Vaquero J, Rose C, Butterworth RF. Keeping cool in acute liver failure: rationale for the use of mild hypothermia. J Hepatol 2005;43:1067–1077.
Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology 2004;127:1338–1346.
Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med 2007;35:2498–2508.
Acknowledgements
Dr. Muehlschlegel has received grant funding from the following entities:
American Heart Association (Scientist Development Grant No. 09SDG2030022);
Worcester Research Foundation Grant 2010; Faculty Scholar Award 2011 (University of Massachusetts).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 499 kb)
Rights and permissions
About this article
Cite this article
Rivera-Lara, L., Zhang, J. & Muehlschlegel, S. Therapeutic Hypothermia for Acute Neurological Injuries. Neurotherapeutics 9, 73–86 (2012). https://doi.org/10.1007/s13311-011-0092-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-011-0092-7