Abstract
The aim of our study was to assess and compare postoperative nausea and pain after one anastomosis gastric bypass (OAGB) and sleeve gastrectomy (LSG). Patients undergoing OAGB and LSG at our institution between November 2018 and November 2021 have been prospectively asked to report postoperative nausea and pain on a numeric analogic scale. Medical records were retrospectively reviewed to collect scores of these symptoms at the 6th and 12th postoperative hour. One-way analysis of variance (ANOVA) was used to evaluate effect of type of surgery on postoperative nausea and pain scores. To adjust for baseline differences between cohorts, a propensity score algorithm was used to match LSG patients to MGB/OAGB patients in a 1:1 ratio with a 0.1 tolerance. A total number of 228 (119 SGs and 109 OAGBs) subjects were included in our study. Nausea after OAGB was significantly less severe than after LSG both at the 6th and 12th hour assessment; pain was less strong after OAGB at the 6th hour but not after 12 h. Fifty-three individuals had a rescue administration of metoclopramide after LSG and 34 after OAGB (44.5% vs 31.2%, p = 0.04); additional painkillers were required by 41 patients after LSG and 23 after OAGB (34.5% vs 21.1%, p = 0.04). Early postoperative nausea was significantly less severe after OAGB, while pain was comparable especially at the 12th hour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The laparoscopic sleeve gastrectomy (LSG) is currently the most common bariatric procedure worldwide [1], while the one anastomosis gastric bypass (OAGB) represents the third intervention in Europe [2].
Although there is a rich body of literature on postoperative nausea and vomit (PONV) after LSG [3], very little is available on OAGB.
Assessment of these symptoms is particularly important since PONV occurs more frequently after bariatric surgery than after other abdominal surgical procedures [4,5,6].
Moreover, PONV is responsible for prolonged hospital stay and 30-day readmission [7, 8]
Among all weight loss interventions, LSG is undoubtedly the most emetogenic [9] one, probably due to the high intraluminal pressure in the sleeved stomach.
Another main issue in the first postoperative hours is pain. Forty percent of patients with morbid obesity complains with severe postoperative pain which can lead to pulmonary complications and increased risk of thromboembolism due to immobility [10, 11].
To reduce the rate of PONV and the severity of postoperative pain, a specific Early Recovery After Bariatric Surgery (ERABS) protocol has been designed [12, 13].
In our institution, ERABS guidelines are not strictly followed, indeed a nasogastric tube is routinely used and total intravenous anaesthesia (TIVA), instead of volatile anaesthetics, is not given to all patients. However, subjects with morbid obesity undergoing bariatric surgery in our hospital receive the same perioperative management.
The aim of this study is to retrospectively compare postoperative nausea and pain after OAGB and LSG.
Methods
All subjects that have undergone primary OAGB and LSG between 1st November 2018 and 1st November 2021 at our university hospital were included in this study. Those individuals who had an early complication or a concomitant procedure (hiatal hernia repair, abdominal wall reconstruction, cholecystectomy), with previous history of abdominal surgery or transferred to the Intensive Care Unit (ICU) immediately after surgery were excluded.
Patients undergoing bariatric surgery at our institution are routinely asked to report their pain and nausea on a numeric scale (0 = not at all to 10 = worst imaginable nausea/pain).
Medical records were retrospectively reviewed to collect data on preoperative sex, age, body mass index (BMI), pain and nausea at the 6th and 12th postoperative hour and preoperative symptoms of gastro-esophageal reflux disease (GERD).gastro-esophageal reflux disease (GERD) was diagnosed according to the Lyon Consensus Conference [14] criteria in case of preoperative heartburn and regurgitation. Individuals with esophagitis > B according to the Los Angeles Classification [15] are submitted to Roux-en-Y Gastric Bypass (RYGB).
The choice of the procedure (OAGB or LSG) in our centre is based on patients’ BMI and obesity related diseases. Those individuals with higher BMI or metabolic complications such as Diabetes are more likely to undergo OAGB.
Surgical technique
Surgical techniques for both procedures have been described in detail elsewhere [16, 17],but a brief description is reported below for completeness of the article. Patient was placed in the reverse Trendelenburg position.
For LSG, a five-trocar approach (3 × 12 mm, 2 × 5 mm) was used. The gastrectomy started 4–6 cm from the pylorus over a 38–40 French bougie. Staple line reinforcements or oversewing is not routinely used at our institution.
OAGB was routinely performed with a six-port laparoscopic technique. The gastric pouch was constructed by applying one horizontal 45-mm linear stapler at the lesser curvature, just below the left branch of the crow’s foot. Biliopancreatic limb length ranged from 180 to 220 cm depending on the preoperative BMI of patients. All the anastomoses were performed at least 13 cm distally to the GEJ.
Anaesthesia and Postoperative care
After admission to the operating room two intravenous cannulas (16/18-gauge) were inserted; patients were monitored using a five-lead ECG, invasive arterial pressure monitoring, pulse oximeter, capnograph, end-tidal anaesthetic gas (ETAG) concentration monitoring, urine output and temperature. After proper assessment of the airway and anticipation of difficult airway, preoxygenation with 100% O2 on 8 L/min for 3 min via face mask in ramped position was started. Induction was performed with propofol 2 mg/Kg of lean body weight, fentanyl 2–5 mcg/kg of lean body weight, rocuronium 0.6 mg/kg of ideal body weight followed by intubation. Muscle relaxation was monitored through train-of-four (TOF). Ventilation was performed with tidal volumes of 6–8 mL/Kg to avoid barotraumas and respiratory rates 12–14 breaths/min to maintain normocapnia and Positive End Expiratory Pressure (PEEP) of 5–10 cmH2O. Anaesthesia was maintained with desflurane with a MAC between 0.6 and 1, remifentanil infusion of 0.05–0.25 mcg/kg/min of lean body weight. No abdominal wall block or intraoperative injection of local anaesthetics was performed. Postoperative analgesia was provided with a continuous intravenous administration of ketorolac 90 mg, ondansetron 8 mg and oxycodone 10 mg through an elastomeric infusion pump. All patients assumed Paracetamol 1 g iv every 6 h for 24 as a rescue drug. Intravenous metoclopramide 10 mg was administered in case of severe nausea.
A nasogastric tube was placed and left for the first 24 h after both interventions; liquid diet was started on postoperative day (POD) 3. Subjects were mobilized on POD1.
Statistical analysis
Data are expressed as mean ± SD for continuous variables and as proportion or percentage in case of categorical ones. Continuous and categorical variables were compared using the chi-square and t-test, respectively. One-way analysis of variance (ANOVA) was used to evaluate effect of type of surgery on postoperative nausea and pain scores. To adjust for baseline differences between cohorts, a propensity score algorithm was used to match LSG patients to MGB/OAGB patients in a 1:1 ratio with a 0.1 tolerance. Propensity score matching (PSM) is a well-validated statistical technique that creates comparable groups and allows for accurate assessment of treatment effects. Patients were matched for preoperative age, BMI and GERD.
Significant p value was set below 0.05. Data analyses were performed using Statistical Package for Social Science for Windows, version 28 (SPSS Inc., Chicago, IL, USA).
Results
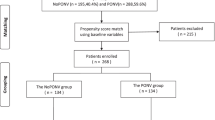
A total number of 136 primary OAGBs and 178 primary SGs have been performed at our institution in the study period. On the base of exclusion/inclusion criteria, 51 cases (early complication or concomitant procedure) were not eligible for this study, while data on postoperative nausea and pain were not available for 35 patients. Subsequently, 228 (119 SGs and 109 OAGBs) subjects were included in our retrospective comparison. After PSM, two matched groups of 73 subjects each were generated.
Patients undergoing the two procedures had comparable age, rate of GERD and female/male ratio at baseline, but BMI was significantly higher in the OAGB group; all preoperative variables resulted comparable at baseline between the matched groups (Table 1).
Overall values of nausea at the 6th and 12th hour were 5.8 ± 1.2 and 3.7 ± 1.3 respectively, while total scores for pain were 5.9 ± 1.1 and 3 ± 1.
Nausea after OAGB was significantly less severe than after LSG both at the 6th- and 12th- hour assessment before and after PSM. On the contrary pain was significantly less strong after OAGB at the 6th hour but not after 12 h before PSM, while no significant difference for pain was found between the matched groups (Table 2).
Discussion
Nausea
Postoperative nausea and vomiting negatively affects early hours after LSG with a reported incidence up to 90% [18]. Occurrence of this symptoms is lower after RYGB, especially when ERABS protocol is used [19]. Even if PONV is usually self-limiting, when this condition persists it has a negative impact on patient satisfaction, hospital stay and risk of readmission [20].
For these reasons, different combinations of perioperative drugs have been suggested to reduce nausea and vomiting after bariatric surgery [21, 22].
In our experience all patients undergoing LSG and OAGB experienced a variable degree of early nausea. Vomiting was not assessed in our retrospective analysis due to the routine use of a nasogastric tube, which is not recommended by the ERABS guidelines [23] and it could have biased the outcomes. Therefore, in our study only nausea was considered as a symptom of delayed functional recovery of the stomach.
Female sex and use of volatile anaesthetics are usually associated with PONV [24]; in our cohort of patient there was no difference in female/male ratio in the two groups and only desflurane, whose efficacy in bariatric patients is comparable to TIVA [25], was used as a volatile anaesthetic.
As reported in the current literature, our data have further confirmed that nausea tends to significantly reduce in the first 12 postoperative hours. Moreover, our comparison demonstrated that regardless preoperative demographics, this symptom was significantly less severe after OAGB. Indeed, after PSM, nausea was significantly more severe after LSG both at the 6th- and 12th-hour assessment. This finding also correlates with the lower rate of rescue medicines used in the postoperative period after OAGB, which could subsequently be considered as a better option for patients with preoperative GERD.
Pain
Uncontrolled postoperative pain has a detrimental effect on respiratory function, mobility, thromboembolic complications, nausea and vomiting [26]. Unfortunately, management of this symptom is a demanding task in patients with morbid obesity [27].
Moreover, excessive use of painkillers during the early hours after surgery increases the risk of chronic post-surgical pain and opioid dependence [28].
Several strategies, such as transversus abdominis plane (TAP) block [29] or multimodal intraoperative administration of different drugs [11, 30], have been proposed to optimize pain management after bariatric surgery.
In our institution a standardized continuous intravenous injection of painkillers is preferred, and opioids are forbidden as rescue medicines. Before PSM, intensity of this symptom was lower after OAGB at the 6th, but no significant difference was found after matching. Even if the resection and removal of 80% of the stomach induced worse early distress, this was adequately controlled in the first 12 h by our protocol. Indeed, this greater postoperative discomfort after LSG was also demonstrated by the higher rate of additional painkillers administered postoperatively.
Strength and limitations
ERABS guidelines are not strictly followed in our department, therefore a nasogastric tube is routinely placed and oxycodone (elastomeric pump) was administered postoperatively to all patients. Nausea and pain assessments relied totally on a self-reported numeric scale and no validated questionnaire was used. Being this study retrospective, possible confounders and heterogeneity related to preoperative selection bias, which we tried to reduce using PSM. However, this is the first report of these symptoms after OAGB in a large cohort of patients undergoing the same perioperative management.
Conclusion
Early postoperative nausea was significantly more severe after LSG rather than after OAGB with a greater percentage of patients requiring rescue medicines.
Pain was more intense after LSG at the 6th hour with higher rate of additional painkillers administered in this group. Postoperative discomfort was comparable after PSM.
References
Angrisani L, Santonicola A, Iovino P et al (2017) Erratum to: bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. OBES SURG 27:2290–2292. https://doi.org/10.1007/s11695-017-2773-8
Angrisani L, Santonicola A, Iovino P et al (2021) Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO Chapters. OBES SURG 31:1937–1948. https://doi.org/10.1007/s11695-020-05207-7
Pourfakhr P, Aghabagheri M, ZabihiMahmoudabadi H et al (2021) Prophylactic administration of diphenhydramine/acetaminophen and ondansetron reduced postoperative nausea and vomiting and pain following laparoscopic sleeve gastrectomy: a randomized controlled trial. Obes Surg 31:4371–4375. https://doi.org/10.1007/s11695-021-05589-2
Suh S, Helm M, Kindel TL et al (2020) The impact of nausea on post-operative outcomes in bariatric surgery patients. Surg Endosc 34:3085–3091. https://doi.org/10.1007/s00464-019-07058-5
Spaniolas K, Nie L, Moller D et al (2020) A Comprehensive approach for the prevention of nausea and vomiting following sleeve gastrectomy: a randomized controlled trial. Obes Surg 30:4250–4257. https://doi.org/10.1007/s11695-020-04795-8
Groene P, Eisenlohr J, Zeuzem C et al (2019) Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne 14(1):90–95
Berger ER, Huffman KM, Fraker T et al (2018) Prevalence and risk factors for bariatric surgery readmissions: findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg 267(1):122–131
Weingarten TN, Hawkins NM, Beam WB et al (2015) Factors associated with prolonged anesthesia recovery following laparoscopic bariatric surgery: a retrospective analysis. Obes Surg 25(6):1024–1030
Kushner BS, Freeman D, Sparkman J, Salles A, Eagon JC, Eckhouse SR (2020) Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg Obes Relat Dis 16(10):1505–1513. https://doi.org/10.1016/j.soard.2020.05.017
Andersen LPH, Werner MU, Rosenberg J et al (2014) Analgesic treatment in laparoscopic gastric bypass surgery: a systematic review of randomized trials. Obes Surg 24:462–470. https://doi.org/10.1007/s11695-013-1172-z
Erdogan Kayhan G, Sanli M et al (2018) Comparison of intravenous ibuprofen and acetaminophen for postoperative multimodal pain management in bariatric surgery: a randomized controlled trial. J Clin Anesth 50:5–11. https://doi.org/10.1016/j.jclinane.2018.06.030
Díaz-Vico T, Cheng YL, Bowers SP, Arasi LC, Chadha RM, Elli EF (2021) Outcomes of enhanced recovery after surgery protocols versus conventional management in patients undergoing bariatric surgery. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2020.0783
Monte SV, Rafi E, Cantie S, Wohaibi E, Sanders C, Scovazzo NC (2021) Reduction in opiate use, pain, nausea, and length of stay after implementation of a bariatric enhanced recovery after surgery protocol. Obes Surg 31(7):2896–2905. https://doi.org/10.1007/s11695-021-05338-5. (Epub 2021 Mar 12 PMID: 33712934)
Gyawali CP, Kahrilas PJ, Savarino E et al (2018) Modern diagnosis of GERD: the lyon consensus. Gut 67(7):1351–1362
Armstrong D, Bennett JR, Blum AL et al (1996) The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 111(1):85–92. https://doi.org/10.1053/gast.1996.v111.pm8698230. (PMID: 8698230)
Vitiello A, Berardi G, Velotti N, De Palma GD, Musella M (2020) Should sleeve gastrectomy be considered only as a first step in super obese patients? 5-year results from a single center. Surg Laparosc Endosc Percutan Tech 31(2):203–207. https://doi.org/10.1097/SLE.0000000000000866. (PMID: 32956334)
Musella M, Vitiello A, Berardi G et al (2020) Evaluation of reflux following sleeve gastrectomy and one anastomosis gastric bypass: 1-year results from a randomized open-label controlled trial. Surg Endosc. https://doi.org/10.1007/s00464-020-08182-3
Fathy M, Abdel-Razik MA, Elshobaky A, Emile SH, El-Rahmawy G, Farid A et al (2019) Impact of pyloric injection of magnesium sulfate-lidocaine mixture on postoperative nausea and vomiting after laparoscopic sleeve gastrectomy: a randomized-controlled trial. Obes Surg 29(5):1614–1623 (PubMed PMID:30734195)
Major P, Stefura T, Małczak P et al (2018) Postoperative care and functional recovery after laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass among patients under ERAS protocol. Obes Surg 28(4):1031–1039. https://doi.org/10.1007/s11695-017-2964-3
Eberhart LH, Mauch M, Morin AM et al (2002) Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia 57:1022–1027
Benevides ML, Oliveira SSDS, de Aguilar-Nascimento JE (2013) The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg 23:1389–1396. https://doi.org/10.1007/s11695-013-0923-1
Kruthiventi SC, Hofer RE, Warner ME, Sprung J, Kellogg TA, Weingarten TN (2020) Postoperative nausea and vomiting after bariatric surgery and dexmedetomidine anesthetic: a propensity-weighted analysis. Surg Obes Relat Dis 16(4):545–553. https://doi.org/10.1016/j.soard.2020.01.007. (Epub 2020 Jan 18 PMID: 32063491)
Thorell A, MacCormick AD, Awad S et al (2016) Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 40:2065–2083. https://doi.org/10.1007/s00268-016-3492-3
Gan TJ, Belani KG, Bergese S et al (2020) Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 131(2):411–448. https://doi.org/10.1213/ANE.0000000000004833. (Erratum in: Anesth Analg. 2020 Nov; 131(5):e241. PMID: 32467512)
Aftab H, Fagerland MW, Gondal G et al (2019) Pain and nausea after bariatric surgery with total intravenous anesthesia versus desflurane anesthesia: a double blind, randomized, controlled trial. Surg Obes Relat Dis 15(9):1505–1512. https://doi.org/10.1016/j.soard.2019.05.010. (Epub 2019 May 14 PMID: 31227317)
Omar I, Abualsel A (2019) Efficacy of intraperitoneal instillation of bupivacaine after bariatric surgery: randomized controlled trial. Obes Surg 29:1735–1741. https://doi.org/10.1007/s11695-019-03775-x
Lloret-Linares C, Lopes A, Declèves X et al (2013) Challenges in the optimisation of post-operative pain management with opioids in obese patients: a literature review. Obes Surg 23(1458):1475
Tian C, Maeda A, Okrainec A et al (2020) Impact of preoperative opioid use on health outcomes after bariatric surgery. Surg Obes Relat Dis 16:768–776
Tian C, Lee Y, Oparin Y et al (2021) Benefits of transversus abdominis plane block on postoperative analgesia after bariatric surgery: a systematic review and meta-analysis. Pain Phys 24(5):345–358 (PMID: 34323436)
Ma P, Lloyd A, McGrath M et al (2019) Efficacy of liposomal bupivacaine versus bupivacaine in port site injections on postoperative pain within enhanced recovery after bariatric surgery program: a randomized clinical trial. Surg Obes Relat Dis 15(9):1554–1562. https://doi.org/10.1016/j.soard.2019.06.004. (Epub 2019 Jun 17 PMID: 31375443)
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. No funding was received to write this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Antonio Vitiello, Carmine Iacovazzo, Givoanna Berardi, Maria Vargas, Annachiara Marra, Pasquale Buonanno, Nunzio Velotti and Mario Musella declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vitiello, A., Iacovazzo, C., Berardi, G. et al. Propensity score matched analysis of postoperative nausea and pain after one anastomosis gastric bypass (MGB/OAGB) versus sleeve gastrectomy (SG). Updates Surg 75, 1881–1886 (2023). https://doi.org/10.1007/s13304-023-01536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01536-1