Abstract
Introduction
This study aimed to understand the actual status of multimorbidity and polypharmacy among patients with type 2 diabetes using glucose-lowering drugs, and to assess the effects of patient characteristics on severe hypoglycemia and glycemic control.
Methods
We designed a retrospective cohort study using health insurance claims and medical checkup data in Japan from April 2016 to February 2021 and identified patients with type 2 diabetes who were prescribed glucose-lowering drugs. We analyzed data on patient characteristics, including multimorbidity and polypharmacy, calculated the incidence rate for severe hypoglycemic events, applied a negative binomial regression model to explore factors that affected severe hypoglycemia, and analyzed the status of glycemic control in the subcohort for which HbA1c data were available.
Results
Within the analysis population (n = 93,801), multimorbidity was present in 85.5% and mean ± standard deviation for oral drug prescriptions was 5.6 ± 3.5 per patient, while for those aged 75 years or older these numbers increased to 96.3% and 7.1 ± 3.5, respectively. The crude incidence rate for severe hypoglycemia was 5.85 (95% confidence interval 5.37, 6.37) per 1000 person-years. Risk factors for severe hypoglycemia included younger and older age, prior severe hypoglycemia, use of insulin, sulfonylurea, two-drug therapy including sulfonylurea or glinides, three-or-more-drug therapy, excessive polypharmacy, and comorbidities including end-stage renal disease (ESRD) requiring dialysis. Subcohort analysis (n = 26,746) showed that glycemic control is not always maintained according to guidelines.
Conclusion
Patients with type 2 diabetes, particularly older patients, experienced high multimorbidity and polypharmacy. Several risk factors for severe hypoglycemia were identified, most notably younger age, ESRD, history of severe hypoglycemia, and insulin therapy.
Trial Registration
The University Hospital Medical Information Network Clinical Trials Registry (UMIN000046736).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In patients with diabetes, multimorbidity is likely to lead to hypoglycemia and polypharmacy, is associated with poor prognosis, and requires careful management. |
Multimorbidity is a known risk factor for severe hypoglycemia, along with prior severe hypoglycemia and use of some hypoglycemic drugs, although the impact of other factors, including the use of other hypoglycemic drugs and effects of polypharmacy, has not yet fully elucidated. |
This study was designed to use real-world data from Japanese health insurance claims to clarify the actual situation regarding multimorbidity and polypharmacy in patients with type 2 diabetes, and to understand the relationship to the risk of severe hypoglycemia in such patients. |
What was learned from the study? |
Patients with type 2 diabetes in Japan tend to experience multimorbidity and polypharmacy, suggesting substantial pill burden, especially among older patients in actual clinical practice. |
Major risk factors for severe hypoglycemia were younger age, end-stage renal disease with dialysis, history of severe hypoglycemia, and insulin therapy; excessive polypharmacy was also identified as a risk factor. |
Introduction
The association between aging and increased comorbidity with chronic diseases has been well documented [1, 2]. Now interest in multimorbidity (co-occurrence of two or more chronic health conditions) is mounting in the field of diabetes. Because multimorbidity tends to lead to hypoglycemia and polypharmacy [3], which can worsen patient prognosis [4], it requires attentive management. Notably, around the world 19.3% of people aged 65 or older have diabetes [5], which makes diabetes management in the older population a challenge to the future of public health. The problem of multimorbidity is an even more critical challenge in this population. Older patients with diabetes tend to have a high incidence of cardiovascular comorbidity and geriatric syndromes, including dementia and urinary incontinence [6], and are thus more prone to experience multimorbidity.
Polypharmacy also warrants attention, since it is associated with an increased risk for drug interactions and adverse events, poor medication adherence, and negative effects on quality of life (QOL) [7,8,9]. But only limited clinical epidemiology data on multimorbidity and polypharmacy in type 2 diabetes have been reported from countries outside of Europe and the USA.
Multimorbidity and polypharmacy are also interesting in the field of diabetes from the aspect of the impact on severe hypoglycemia. Linked to cardiovascular disease (CVD), death [10, 11], and a decrease in patient QOL [12], severe hypoglycemia is associated with high medical care costs and tends in particular to adversely affect older patients and those with comorbidities [13]. Previous studies have shown that the risk of severe hypoglycemia is associated with factors including the number of comorbidities, prior severe hypoglycemia, use of sulfonylurea (SU) or insulin therapies, and intensive glycemic control [13, 14]. However, researchers have not yet fully elucidated the impact of other factors, including the use of other hypoglycemic drugs and polypharmacy. Effective clinical decision-making absolutely requires a more thorough understanding of these factors to reduce the risk of severe hypoglycemia and improve patient QOL.
For glycemic control, the following target values are recommended worldwide: hemoglobin A1c (HbA1c) goal of 7.0% for the majority of adult patients [15,16,17]; and a “less stringent” HbA1c goal of 8.0% for patients with limited life expectancy or for whom the harms of treatment outweigh the benefits [16]. In older patients, safety-minded appropriate glycemic control is recommended, and glycemic goals are set at 7.0–7.5% for healthy people and 8.0% for patients with multiple comorbidities [6, 17]. However, previous studies have revealed overtreatment in older patients or in patients with serious comorbidities [18, 19], and young adult patients have shown poorer glycemic control than older patients [20, 21]. To achieve better diabetes management globally, we must thus first understand real-world treatment in individual countries and regions within multiple racial and healthcare environments.
In this study we examined Japanese real-world data to understand the actual situation regarding multimorbidity, polypharmacy, and prescription of hypoglycemic drugs in patients with type 2 diabetes and to investigate the impact of patient characteristics on severe hypoglycemia. We also examined the status of glycemic control in those patients.
Methods
Study Design and Setting
This study was a retrospective cohort study using health insurance claims and medical checkup data from April 2016 to February 2021, with data sourced from DeSC Healthcare Inc. (Tokyo, Japan). The data consist of anonymously processed healthcare-related information from insurance claims, including diagnostic codes, prescription and procedure codes, and medical checkups. Individual healthcare information is traceable across all Japanese medical institutions as long as members continue to belong to the same health insurance society [22]. Japanese health insurance societies provide subscribers with annual medical checkups to confirm the health status of each person. That medical checkup data is available for about one-third of the entire population in this database. The three insurance societies providing the data for this database were the Society-managed, Employment-based Health Insurance association (SHI), serving individuals working at large companies and their family members, the National Health Insurance (NHI), serving people younger than 75 who are not covered by other public health systems, such as self-employed persons, farmers, college students, and retirees, and the Latter-Stage Elderly Healthcare System (LSEHS), serving people aged 75 years or over [23]. Together, these societies cover over three million individuals in Japan. The study was registered through the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000046736).
Because the data in this study were fully anonymized, coming from a health insurance claims database and medical checkup data, there was no need to obtain informed consent from individual patients or approval from the institutional review board (IRB). The data were purchased from DeSC Healthcare Inc., and permission was obtained to use them in the study and to publish the results in a scientific paper. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Participants
Patients with type 2 diabetes who were prescribed glucose-lowering drugs on an outpatient basis during the enrolment period of April 2017 to February 2020 were identified. The index date was defined as the date that the prescription started for outpatient care, and the index month was defined as the month including the index date. Patients who were 18 years of age or older at the index month and had been continuously registered in the database for the year preceding the index month (baseline period) were included in the analysis population. We excluded from analysis patients with type 1 diabetes during the baseline period, including the index month. The definitions of diseases and drugs are presented in Supplemental Table S1.
Study Measures and Definitions
Endpoints included patient characteristics, the occurrence of severe hypoglycemia, and glycemic control.
Patient comorbidities during the baseline period were considered to be any of the following 16 health conditions [13, 24], as specified in the guidelines [6, 25, 26] and consensus report [27]: dementia, end-stage renal disease (ESRD; dialysis), chronic kidney disease (CKD; excluding dialysis), myocardial infarction, congestive heart failure (CHF), cerebrovascular disease (CBVD), chronic obstructive pulmonary disease (COPD), malignancy (excluding malignant skin neoplasm), cirrhosis, diabetic retinopathy, diabetic neuropathy, hypertension, arthritis, urologic diseases or conditions, depression, and fracture. These conditions are defined in Supplemental Table S1. Multimorbidity was defined as the presence of type 2 diabetes and at least one of the conditions listed above. Polypharmacy was calculated on the basis of the number of prescription drugs (oral medications that were prescribed for at least 7 days including the index date, except for anti-infective agents other than antiviral agents). A total of ≥ 5 to ≤ 9 prescription drugs was defined as polypharmacy, and ≥ 10 as excessive polypharmacy. Prior severe hypoglycemia was defined as at least one occurrence of severe hypoglycemia during the baseline period. Severe hypoglycemia was defined as a prescription for either 20%, 40%, or 50% glucose infusion or glucagon infusion recorded on a claims form related to hypoglycemia (Supplemental Table S1). In the category of glucose-lowering drugs, drugs were identified that were prescribed on the index date (biguanides, dipeptidyl peptidase 4 inhibitors [DPP4i], glucagon-like peptide 1 [GLP-1] receptor agonists, sodium-glucose cotransporter 2 inhibitors [SGLT2i], thiazolidines, α-glucosidase inhibitors, glinides, sulfonylurea [SU], basal insulin, and other types of insulin). Glucose-lowering drug therapies were classified into monotherapy, combination therapy with two drugs, or combination therapy with three or more drugs. Prior glucose-lowering therapy was defined as previously treated (PT) if glucose-lowering drugs were prescribed during the baseline period, or as previously untreated (UT) if they were not.
Statistical Methods
For patient characteristics, we calculated the number and percentage of patients for categorical variables and the mean value and standard deviation (SD) for continuous variables. To describe patient characteristics, we analyzed subgroups based on prior glucose-lowering therapy (PT or UT), the age category, and the glucose-lowering drug prescribed on the index date.
For severe hypoglycemia, we calculated the total duration of follow-up, the number of occurrences, and the crude (unadjusted) incidence rate and 95% confidence interval (CI). The incidence rate was calculated as the number of occurrences of severe hypoglycemia per 1000 person-years. The follow-up period was a maximum of 1 year from the index date. Crude incidence rates were calculated by individual patient characteristics, including age, sex, comorbidity, multimorbidity, and polypharmacy. The main analysis for the factors that affect severe hypoglycemia was conducted using a negative binomial regression model with log-transformed follow-up time as an offset. We performed both univariable and multivariable analysis, with age, sex, comorbidity, glucose-lowering drugs, polypharmacy, prior severe hypoglycemia, and prior glucose-lowering therapy as explanatory variables, and with the number of occurrences of severe hypoglycemia as objective variables. Results were reported as incidence rate ratios (IRRs) and 95% CIs for severe hypoglycemia. For sensitivity analysis we used a multivariable analysis method with the same definition of severe hypoglycemia as described above, except that we excluded prescriptions of 20% and 40% glucose infusions. We also used a logistic regression model for multivariable analysis of the occurrence of severe hypoglycemia.
Glycemic control status was investigated in a subcohort having HbA1c data that were collected in a health checkup 28 days or more after the index date. If multiple HbA1c data were available, the data closest to the index date were used. Patient characteristics were calculated using data from 1 year previous to HbA1c measurement. Descriptive statistics of HbA1c were calculated for the entire analysis population and for each patient characteristic, and the proportion of achievement of the adult HbA1c target (< 7.0%) was calculated. Subgroup analysis was based on age group and age plus multimorbidity status in patients with glucose-lowering drugs. HbA1c values were classified into seven categories (< 6.0%, ≥ 6.0% to < 6.5%, ≥ 6.5% to < 7.0%, ≥ 7.0% to < 7.5%, ≥ 7.5% to < 8.0%, ≥ 8.0% to < 8.5%, and ≥ 8.5%), and the numbers and percentages of patients were calculated for each category. All statistical analyses were performed using SAS version 9.4.
Results
Analysis Population
Patient disposition is shown in Supplemental Fig. S1. During the period from April 2017 to February 2020, we identified 93,801 patients who met the eligibility criteria for the analysis population.
Patient Characteristics
Table 1 summarizes patient characteristics except for comorbidities and prescribed glucose-lowering drugs. The full analysis population (n = 93,801) was 67.2 ± 12.3 years of age and consisted of 60.0% men. All patients belonged to one of the three insurance societies. Most patients (93.9%) were treated at clinics having 19 beds or fewer. Within the analysis population, 72,085 (76.8%) were PT and 21,716 (23.2%) were UT. Patients with multimorbidity accounted for 85.5% of the analysis population. The mean number of comorbidities in addition to diabetes was 3.2 ± 1.7. PT patients showed a higher proportion of multimorbidity than UT patients (90.7% vs. 68.3%). Analysis by age category showed age-related increases in the proportion of multimorbidity and the mean number of comorbidities. In patients 75 years or older, the proportion of multimorbidity was particularly high at 96.3%. Of the full analysis population, 52.4% were receiving monotherapy with a glucose-lowering drug, 26.0% were receiving two-drug combination therapy, and 21.6% were receiving combination therapy with three or more drugs. Monotherapy was used in a higher proportion of UT patients than PT patients (87.6% vs. 41.8%). Patients were prescribed 5.6 ± 3.5 oral drugs, 43.4% of patients met the definition for polypharmacy (a total of ≥ 5 to ≤ 9 prescription drugs), and 12.9% met the definition for excessive polypharmacy (a total of ≥ 10 prescription drugs). Increasing age was associated with a higher percentage of polypharmacy (18–44 years, 25.0%; ≥ 75 years, 53.5%) and of excessive polypharmacy (18–44 years, 6.4%; ≥ 75 years, 22.3%). The mean number of prescribed drugs also increased with age (18–44 years, 3.9 ± 3.3, ≥ 75 years, 7.1 ± 3.5) (Table 1).
Table 2 lists details of patient comorbidities. Of the 16 comorbid conditions, the most common was hypertension (67.6%), followed by CKD (23.0%), CBVD (21.3%), and diabetic retinopathy (20.1%). Diabetic neuropathy was relatively uncommon, accounting for 5.6% of patients. Patients 75 years or older showed a notable increase in a variety of aging-related diseases: hypertension (82.8%), CBVD (38.9%), CHF (33.2%), CKD (32.6%), urologic diseases or conditions (24.6%), COPD (18.0%), malignancy (15.5%), fracture (15.3%), and dementia (14.2%) (Table 2).
Table 3 shows patient characteristics regarding prescribed glucose-lowering drugs. DPP4i was taken by the largest number of patients on monotherapy (31.9%). The most common form of two-drug combination therapies was biguanide plus DPP4i (8.5%). Trends in prescription patterns for glucose-lowering drugs varied by age category. In all age categories, DPP4i was most frequently prescribed, with the percentage of use differing notably between the two age categories of 18–44 years (20.9%) and 75 years or older (41.9%). Prescription proportions for SU increased with age, while glinide was prescribed at a nearly constant level across age groups (Table 3).
Patient characteristics were aggregated by glucose-lowering drug (Supplemental Table S2).
Crude (Unadjusted) Incidence Rates for Severe Hypoglycemia
The incidence rates for severe hypoglycemia are shown in Supplemental Table S3. In this section, all incidence rates are unadjusted. The overall incidence rate was 5.85 (95% CI 5.37, 6.37) per 1000 person-years. By age category, the incidence rates showed a U-shaped pattern with the lowest rate among patients aged 50–59 years (2.70 [1.86, 3.79] per 1000 person-years) and higher rates in patients who were younger or older. For comorbidity, the highest incidence rate was in patients with ESRD (110.31 [92.55, 130.49] per 1000 person-years). Incidence rates for severe hypoglycemia rose with increasing numbers of comorbidities. Patients with prior severe hypoglycemia experienced the highest incidence of recurrent events (401.50 [332.05, 481.19] per 1000 person-years). By class of glucose-lowering drug, we found the highest incidence rate for severe hypoglycemia in patients on insulin (52.47 [42.35, 64.28] per 1000 person-years), including basal insulin (39.50 [23.77, 61.69]) and other types of insulin (57.30 [44.99, 71.94]). The incidence rate for severe hypoglycemia was higher in the categories of polypharmacy (5.65 [4.93, 6.43] per 1000 person-years) and excessive polypharmacy (17.43 [15.12, 20.00]) than of no polypharmacy (2.69 [2.21, 3.25]).
Factors that Influence the Incidence of Severe Hypoglycemia
Figure 1 and Supplemental Table S4 show results from the main analysis for factors affecting the incidence rate of severe hypoglycemia. Multivariable analysis identified risk factors in multiple subcategories: age ≤ 29, 40–49, 70–79, and ≥ 80; dementia; ESRD; CKD; CHF; malignancy; cirrhosis; fracture; prior severe hypoglycemia; SU; insulin; SU combination (two-drug combination including SU); glinide combination (two-drug combination including glinide); basal insulin-supported oral therapy (BOT); other types of insulin combination (two-drug combination including other types of insulin); three-or-more-drug combination; and excessive polypharmacy.
Factors affecting the incidence rate of severe hypoglycemia. The explanatory variables that were included in the negative binomial regression model were age, sex, comorbidity, glucose-lowering drugs, polypharmacy, prior severe hypoglycemia, and prior glucose-lowering therapy at baseline, with log-transformed follow-up time as an offset. The IRR was calculated for each comorbidity, using the absence of that comorbidity as the reference value (not shown here). If no hypoglycemic episodes were recorded, data for that category were not analyzed. The forest plot shows the point estimate and 95% CI for the IRR in multivariable analysis. aBasal insulin, other types of insulin. bSU + biguanide, DPP4i, SGLT2i, glinide, GLP-1RA, thiazolidine, α-glucosidase inhibitor. cGlinide + biguanide, DPP4i, SGLT2i, GLP-1RA, thiazolidine, α-glucosidase inhibitor. dBasal insulin + biguanide, DPP4i, SGLT2i, glinide, SU, thiazolidine, α-glucosidase inhibitor. eOther types of insulin + biguanide, DPP4i, SGLT2i, glinide, SU, GLP-1RA, thiazolidine, α-glucosidase inhibitor, basal insulin. fDPP4i + biguanide, SGLT2i, GLP-1RA, thiazolidine, α-glucosidase inhibitor. gTwo drugs including biguanide, SGLT2i, GLP-1RA, thiazolidine, α-glucosidase inhibitor. BOT basal insulin-supported oral therapy, CBVD cerebrovascular disease, CHF congestive heart failure, CI confidence interval, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, DPP4i dipeptidyl peptidase 4 inhibitor, ESRD end-stage renal disease, GLP-1RA glucagon-like peptide 1 receptor agonist, IRR incidence rate ratio, SGLT2i sodium glucose cotransporter 2 inhibitor, SU sulfonylurea
The results from sensitivity analysis, based on the modified definition of severe hypoglycemic events, were similar to those from the main analysis, but those results also suggested that age 30–39, CBVD, diabetic retinopathy, arthritis, and glinide use affect the incidence of severe hypoglycemia (Supplemental Fig. S2). Multivariable logistic analysis also supported the influence of CBVD, diabetic retinopathy, and glinide, but indicated that the influence of cirrhosis was diminished (Supplemental Fig. S3).
Status of Glycemic Control
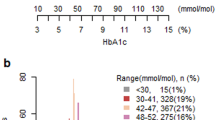
Results of the status of glycemic control in the subcohort for whom HbA1c data were available (n = 26,746) are shown in Supplemental Table S5. Overall, that subcohort had a mean age of 65.0 ± 11.6 years. Patients were predominately male (66.2%), and 85.6% had multimorbidity. Mean HbA1c was 6.87 ± 0.95%. The target HbA1c of < 7.0% was achieved in 63.7% of subjects and was higher in women than in men (67.4% vs. 61.8%). HbA1c values correlated inversely with age: HbA1c decreased as age increased. Target achievement proportion was higher in older than in younger populations (72.8% for age ≥ 75 years vs. 50.9% for age 18–44 years). When we aggregated results by comorbidity, we noted that target achievement proportion tended to be lower in patients with diabetic retinopathy (54.9%) and patients with diabetic neuropathy (53.4%), and higher in patients with more comorbidities. Results stratified by glucose-lowering drug showed a lower proportion of target achievement in patients on basal insulin or other types of insulin (29.2% and 43.9%, respectively) within the category of monotherapy. In comparison to monotherapy, combination therapies were associated with lower target achievement (monotherapy 74.7%, two drugs 59.7%, three or more drugs 44.1%). Subgroup analyses were stratified by age and presence of multimorbidity to investigate the status of glycemic control (Fig. 2). In the subgroup below 65 years of age, 42.9% of patients had HbA1c higher than the target of < 7%. Glucose was controlled to less than 6.5% for a higher percentage of patients in the subgroups aged 65 years or older and 75 years or older than in the subgroup aged 18 to 64 years (Fig. 2a). In the subgroup aged 65 years or older, HbA1c was controlled at 7.0–7.5% in 19.4% of patients without multimorbidity and at 7.5–8.0% in 8.0% with multimorbidity (Fig. 2b).
Status of glycemic control (subgroup analysis). a Distribution of HbA1c, stratified by age. b Distribution of HbA1c in patients aged 65 years or older, stratified by multimorbidity. Subgroup analysis was based on age group and age plus multimorbidity status in patients who had been prescribed glucose-lowering drugs. HbA1c hemoglobin A1c
Discussion
This study focused on the status of multimorbidity and polypharmacy in 93,801 patients with type 2 diabetes who were prescribed glucose-lowering drugs, using health insurance claims data in Japan. This is the first study in Japan to report on multimorbidity and polypharmacy in a large-scale real-world data set of patients with type 2 diabetes, including those aged 65 years or older. The data showed that patients with type 2 diabetes experienced high levels of multimorbidity and received multiple medications. Within the overall analysis population, multimorbidity was noted in 85.5% of patients, with a mean of 5.6 oral drugs per patient. The proportion of patient comorbidities was practically consistent with previous reports [13, 28], except that the proportion of diabetic neuropathy was unexpectedly low (5.6%) in our population. This could be because the condition was not correctly diagnosed in some patients, as diabetic neuropathy can present as a wide variety of clinical signs and symptoms, and effective treatments are limited [29].
Increasing age was related to higher levels of polypharmacy, with 7.1 ± 3.5 oral drugs per patient 75 and older, suggesting that these patients have a heavy pill burden. Particularly when we consider the effects of declining physiological function [30], these findings underscore the problems inherent in polypharmacy for this vulnerable patient population. One possible approach is to reduce the number of therapeutic drugs for each condition whenever possible. In the field of diabetes, for example, although glycemic control should be carefully managed, such control must be balanced with the need to address polypharmacy by switching to monotherapy, drugs with lower dosing frequency, and/or fixed-dose combination therapy.
Our data showed that DPP4i was the most widely prescribed glucose-lowering drug among patients taking monotherapy, which reflected a strong trend toward DPP4i prescription reported in Japan [31, 32], whereas European and US guidelines at the time of this study recommended the use of metformin along with first-line treatment [33, 34]. We also found that the pattern for prescribing glucose-lowering drugs varied according to patient age. With increasing age, DPP4i prescriptions tend to increase and SGLT2i prescriptions tend to decrease. Additionally, in patients 75 and older, the use of monotherapy tended to be higher (Table 1). These findings may reflect the guideline’s recommendations for less strict glycemic control that places more value on safety in the older population [6, 17, 35].
We also considered adjusted relationships between patient characteristics and occurrence of severe hypoglycemia. Risk factors for severe hypoglycemia were younger and older age; comorbidity of ESRD, malignancy, dementia, cirrhosis, CKD, fracture, or CHF; prior severe hypoglycemia; treatment with other types of insulin combination, insulin, BOT, three-or-more-drug combination, glinide combination, SU, or SU combination; and excessive polypharmacy. Both sensitivity analysis based on a modified definition of severe hypoglycemia and analysis of factors that influenced the occurrence of severe hypoglycemia yielded similar trends, demonstrating that our results were robust. We highlighted in this study that younger age, ESRD with dialysis, history of severe hypoglycemia, and insulin therapy were major risk factors for hypoglycemia, emphasizing the need for particularly careful glucose control to avoid hypoglycemia in these vulnerable patient populations.
We noted a U-shaped relationship between age and severe hypoglycemia, similar to findings from a previous study [36] that reported higher rates of hypoglycemic hospitalization in patients below 40 and over 70 years old. Older patients are generally considered more prone to hypoglycemia because of complications associated with aging, but younger patients may not always adhere to the recommended self-care practices for glycemic control [37].
Insulins and SU have been previously reported as risk factors for severe hypoglycemia [13], and glinides are a known risk specifically among patients on dialysis [38]. Our study also showed increased risk for severe hypoglycemia in patients on insulins, SU, or glinides. In addition, we found a higher proportion of ESRD in patients on monotherapy with insulin or glinide or on two-drug combination therapy including glinide (Supplemental Table S2). Polypharmacy has been reported as a risk for severe hypoglycemia [39, 40]. Our results did not support those findings, but did identify polypharmacy with more than 10 drugs as a risk factor that should be noted in daily practice.
Analysis of the status of glycemic control showed that proportion of target achievement was higher in patients who had more comorbidities and those whose treatment regimen was classed as either polypharmacy or excessive polypharmacy. Perhaps, in patients with comorbidities such as CKD or CVD, treatment for the comorbidity was prioritized over finely tuned glycemic control. These findings may shed light on the current clinical situation, in which glucose-lowering drugs are prescribed as standard procedure without considering the options of dose reduction or discontinuation to reduce the risk of hypoglycemia.
Our analysis by class of glucose-lowering drugs showed that intensive treatment with insulin or a combination of two or more drugs was associated with lower target achievement in patients. However, it should be noted that the baseline HbA1c values were not evaluated in this study, and that the analysis was not controlled for confounding. Notably, intensive glucose-lowering is generally provided for severe disease, and that severity may have increased the difficulty of achieving blood glucose-lowering goals.
In addition, in real-world clinical practice blood glucose levels are not always controlled to the target level recommended by treatment guidelines. HbA1c values correlated inversely with age, showing a decrease in mean HbA1c and an increase in target achievement rate in older patients. Previous studies have shown overtreatment in older patients and those with comorbidities [18, 19, 41], and of poorer glycemic control in young adult patients than in older patients [20, 21]. Those findings were substantiated by our study. Since most of our older patients with multimorbidity had HbA1c controlled at lower levels than the target of < 8.0% [6, 17, 35], and since the incidence of severe hypoglycemia was higher in older patients, we can assume that a relatively high percentage of older patients remained on treatment not considered less stringent. Our findings suggest that, particularly in older patients with risk factors including multimorbidity, blood glucose level should be carefully managed to achieve an individually determined target value.
This study did not investigate patient frailty. However, previous studies have reported that frailty was common in older patients with diabetes [42], that polypharmacy was common [43], and that intensive glycemic control did not lower the risk of CVD or death but did increase the incidence of severe hypoglycemia in frail patients [44]. Those findings suggest that QOL may be improved in older patients with diabetes by reducing the number of drugs.
A strength of this study is the use of large real-world data sources from Japanese health insurance payers to access data from approximately 100,000 patients with type 2 diabetes, enabling assessment of highly generalizable populations including patients aged 65 years or older.
This study has several limitations. First of all, the secondary use of medical information data always involves limited validity in terms of the definitions of diseases and outcomes. In particular, severe hypoglycemia may be underestimated because it is not counted if it does not appear in the form of an insurance claim within the database. However, even without validating the definition of severe hypoglycemia, we were able to obtain similar results from sensitivity analysis with a modified definition of hypoglycemia. Second, no “gold standard” has been established for measuring multimorbidity, and we lack clear comprehensive criteria for the measurement of multimorbidity required for appropriate selection of chronic conditions [3, 45]. In this study, we defined and evaluated 16 chronic health conditions that are associated with diabetes, but the prevalence of chronic conditions would have been different if we had chosen other defining criteria. Third, there was a limitation of generalizability in analysis of glycemic control status, because the medical checkup data were obtained from a portion of the population only, and in particular, older adults covered by the NHI and LSEHS may be healthy subscribers. In addition, glucose was not monitored continuously. However, patient background information in the subcohort for which HbA1c data were available has been confirmed to be sufficiently similar to that in the entire cohort. The fourth limitation was that the numbers of cases for which medical checkup data were available were limited, so we were unable to include laboratory data such as HbA1c as an explanatory factor for severe hypoglycemia. The fifth limitation was possible unknown or unmeasured confounding factors, despite the multivariable analysis performed to adjust for confounding factors for severe hypoglycemia data.
Conclusion
Patients with type 2 diabetes experienced high multimorbidity and polypharmacy, suggesting that they have a large pill burden, especially among older patients. Risk factors for severe hypoglycemia included younger and older age, prior severe hypoglycemia, the use of specific glucose-lowering drugs, excessive polypharmacy, and certain chronic conditions such as ESRD. Our findings emphasized the importance of considering individual patient characteristics in patients with type 2 diabetes, so that each patient’s care can be optimized to reduce the risk of severe hypoglycemia.
References:
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43.
Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS ONE. 2014;9: e102149.
Aoki T, Yamamoto Y, Ikenoue T, Onishi Y, Fukuhara S. Multimorbidity patterns in relation to polypharmacy and dosage frequency: a nationwide, cross-sectional study in a Japanese population. Sci Rep. 2018;8:3806.
Chiang JI, Jani BD, Mair FS, et al. Associations between multimorbidity, all-cause mortality and glycaemia in people with type 2 diabetes: a systematic review. PLoS ONE. 2018;13: e0209585.
International Diabetes Federation IDF Diabetes Atlas, 9th ed. Brussels, Belgium. 2019. https://www.diabetesatlas.org. Accessed 16 Aug 2022.
American Diabetes Association Professional Practice Committee. 13. Older adults: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S195–207.
Pérez-Jover V, Mira JJ, Carratala-Munuera C, et al. Inappropriate use of medication by elderly, polymedicated, or multipathological patients with chronic diseases. Int J Environ Res Public Health. 2018;15:310.
Kojima T, Mizukami K, Tomita N, et al. Screening tool for older persons’ appropriate prescriptions for Japanese: report of the Japan Geriatrics Society Working Group on “Guidelines for medical treatment and its safety in the elderly.” Geriatr Gerontol Int. 2016;16:983–1001.
Huang ES, Karter AJ, Danielson KK, Warton EM, Ahmed AT. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med. 2010;25:141–6.
Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8.
Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340: b4909.
McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19:792–9.
McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control, and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA Netw Open. 2020;3:e1919099.
Silbert R, Salcido-Montenegro A, Rodriguez-Gutierrez R, Katabi A, McCoy RG. Hypoglycemia among patients with type 2 diabetes: epidemiology, risk factors, and prevention strategies. Curr Diab Rep. 2018;18:53.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;2018(61):2461–98.
American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S83-96.
Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11:165–223.
Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174:259–68.
Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–62.
Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40:468–75.
Styles E, Kidney RSM, Carlin C, Peterson K. Diabetes treatment, control, and hospitalization among adults aged 18 to 44 in Minnesota, 2013–2015. Prev Chronic Dis. 2018;15:E142.
Okada A, Yasunaga H. Prevalence of noncommunicable diseases in Japan using a newly developed administrative claims database covering young, middle-aged, and elderly people. JMA J. 2022;5:190–8.
Ministry of Health, Labour and Welfare. An outline of the Japanese medical system. https://www.mhlw.go.jp/english/policy/health-medical/health-insurance/index.html. Accessed 2 Apr 2021.
McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Paradox of glycemic management: multimorbidity, glycemic control, and high-risk medication use among adults with diabetes. BMJ Open Diabetes Res Care. 2020;8: e001007.
Conlin PR, Colburn J, Aron D, Pries RM, Tschanz MP, Pogach L. Synopsis of the 2017 U.S. Department of Veterans Affairs/U.S. Department of Defense clinical practice guideline: management of type 2 diabetes mellitus. Ann Intern Med. 2017;167:655–63.
The Management of Type 2 Diabetes Mellitus in Primary Care Work Group. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care 2017. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Accessed 16 Aug 2022.
Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–56.
Ikeda Y, Kubo T, Oda E, Abe M, Tokita S. Incidence rate and patient characteristics of severe hypoglycemia in treated type 2 diabetes mellitus patients in Japan: retrospective diagnosis procedure combination database analysis. J Diabetes Investig. 2018;9:925–36.
Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938–48.
Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14.
Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9: e025806.
Bouchi R, Sugiyama T, Goto A, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2022;13:280–91.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221–8.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2020;41:255–323.
Sako A, Yasunaga H, Matsui H, et al. Hospitalization for hypoglycemia in Japanese diabetic patients: a retrospective study using a national inpatient database, 2008–2012. Medicine (Baltimore). 2015;94: e1029.
Nanayakkara N, Pease AJ, Ranasinha S, et al. Younger people with type 2 diabetes have poorer self-care practices compared with older people: results from the Australian National Diabetes Audit. Diabet Med. 2018;35:1087–95.
Hsiao CC, Tu HT, Lin CH, Chen KH, Yeh YH, See LC. Temporal trends of severe hypoglycemia and subsequent mortality in patients with advanced diabetic kidney diseases transitioning to dialysis. J Clin Med. 2019;8:420.
Lee SE, Kim KA, Son KJ, et al. Trends and risk factors in severe hypoglycemia among individuals with type 2 diabetes in Korea. Diabetes Res Clin Pract. 2021;178:1–7.
Alão S, Conceição J, Dores J, et al. Hypoglycemic episodes in hospitalized people with diabetes in Portugal: the HIPOS-WARD study. Clin Diabetes Endocrinol. 2021;7:1–12.
Sonmez A, Tasci I, Demirci I, et al. A cross-sectional study of overtreatment and deintensification of antidiabetic and antihypertensive medications in diabetes mellitus: the TEMD Overtreatment Study. Diabetes Ther. 2020;11:1045–59.
Hanlon P, Fauré I, Corcoran N, et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020;1:e106–16.
Gutiérrez-Valencia M, Izquierdo M, Cesari M, Casas-Herrero Á, Inzitari M, Martinez-Velilla N. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84:1432–44.
Nguyen TN, Harris K, Woodward M, et al. The Impact of frailty on the effectiveness and safety of intensive glucose control and blood pressure-lowering therapy for people with type 2 diabetes: results from the ADVANCE trial. Diabetes Care. 2021;44:1622–9.
Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–11.
Acknowledgements
Funding
This study, including the Rapid Service Fee, was supported by Teijin Pharma Limited.
Medical Writing and Editorial Assistance
Review of the statistical analysis plan and statistical analysis was supported by Koji Ashizawa, affiliated with Takumi Information Technology Inc. Medical writing support was provided by EDIT, Inc. (Tokyo, Japan) and was funded by Teijin Pharma Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ruriko Koto drafted the manuscript, and Akihiro Nakajima performed the statistical analysis. Ken Sugimoto confirmed the validity of the study design and interpretation of the results from a medical perspective. All authors participated in the study design and the analysis and/or interpretation of data. All authors have read and approved this final version of the manuscript for submission.
Disclosures
Ruriko Koto, Akihiro Nakajima, and Tetsuya Miwa are employees of Teijin Pharma Limited and hold stock in Teijin Limited. Ken Sugimoto has received consulting fees from Teijin Pharma Limited and payment for presentations from Sumitomo Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Kyowa Kirin Co., Ltd.
Compliance with Ethics Guidelines
Because the data in this study were fully anonymized, coming from a large health insurance claims database and medical checkup data, there was no need to obtain informed consent from individual patients or approval from the institutional review board (IRB). The data were purchased from DeSC Healthcare Inc., and permission was obtained to use them in the study and to publish the results in a scientific paper. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available due to restrictions on use of the data provided from DeSC Healthcare Inc. (Tokyo, Japan) under the license for the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Koto, R., Nakajima, A., Miwa, T. et al. Multimorbidity, Polypharmacy, Severe Hypoglycemia, and Glycemic Control in Patients Using Glucose-Lowering Drugs for Type 2 Diabetes: A Retrospective Cohort Study Using Health Insurance Claims in Japan. Diabetes Ther 14, 1175–1192 (2023). https://doi.org/10.1007/s13300-023-01421-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01421-5