Abstract
Introduction
The objective of the current study was to assess the long-term cost-effectiveness of once-weekly semaglutide 0.5 mg and 1.0 mg versus dulaglutide 1.5 mg for the treatment of patients with type 2 diabetes uncontrolled on metformin in the Chinese setting.
Methods
The Swedish Institute of Health Economics Diabetes Cohort Model (IHE-DCM) was used to evaluate the long-term health and economic outcomes of once-weekly semaglutide and dulaglutide. Analysis was conducted from the perspective of the Chinese healthcare systems over a time horizon of 40 years. Data on baseline cohort characteristics and treatment effects were sourced from the SUSTAIN 7 clinical trial. Costs included treatment costs and costs of complications. Projected health and economic outcomes were discounted at a rate of 5% annually. The robustness of the results was evaluated through one-way sensitivity analyses and probabilistic sensitivity analyses.
Results
Compared with dulaglutide 1.5 mg, once-weekly semaglutide 0.5 mg and 1.0 mg were associated with improvements in discounted life expectancy of 0.04 and 0.10 years, respectively, and improvements in discounted quality-adjusted life expectancy of 0.08 and 0.19 quality-adjusted life years (QALYs), respectively. Clinical benefits were achieved at reduced costs, with lifetime cost savings of 8355 Chinese Yuan (CNY) with once-weekly semaglutide 0.5 mg and 11,553 CNY with once-weekly semaglutide 1.0 mg. Sensitivity analyses verified the robustness of the research results.
Conclusions
Once-weekly semaglutide was suggested to be dominant (more effective and less costly) versus dulaglutide 1.5 mg in patients with type 2 diabetes uncontrolled on metformin treatment in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
As one of the most prevalent chronic diseases, diabetes places a heavy clinical and economic burden on the health system in China. |
In China, once-weekly semaglutide has been included in the National Reimbursement Drug List through price negotiation in 2021 and was eligible for reimbursement from 1 January 2022, while evidence of its long-term cost-effectiveness in this country is limited. |
Using data from the SUSTAIN 7 clinical trial, the study aims to assess the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide among Chinese patients with type 2 diabetes. |
What was learned from the study? |
Both once-weekly semaglutide 0.5 mg and 1.0 mg are dominant treatment options versus dulaglutide 1.5 mg among patients with type 2 diabetes uncontrolled with metformin in China. Once-weekly semaglutide is associated with improvement in discounted quality-adjusted life years and reduction in lifetime direct medical costs compared with dulaglutide. |
The current study highlights the economic value of once-weekly semaglutide and provides evidence for healthcare decision-making in China. |
Introduction
Diabetes is one of the most prevalent chronic diseases in China, placing a substantial clinical and economic burden on the health system. A national survey of diabetes in mainland China from 2015 to 2017 showed that there were about 129 million people with diabetes in China [1], of whom about 90% had type 2 diabetes (T2D) [2]. However, only 60 million patients were diagnosed [3]. Among the 42 million patients who received anti-diabetes treatment in China, only 21 million of them have achieved the goal of glycemic control [4]. In 2019, the medical expenditure on diabetes accounted for 11.4% of the total medical expenses in China [3, 5]. The onerous economic burden comes mainly from a series of chronic complications caused by long-term poor blood glucose control. A study found that 67% of patients with T2D in China suffered from diabetes-related complications [6]. In particular, 35.2% of patients with T2D suffered from cardiovascular diseases (CVD) [6], and 72% were at risk of CVD [7].
The latest Guideline for Type 2 Diabetes in China (2020) recommends the use of glucagon-like peptide-1 receptor agonist (GLP-1RA) to further improve the blood glucose levels in patients who fail to meet the glycated hemoglobin A1c (HbA1c) targets with lifestyle intervention and metformin treatment [2]. It also recommends that metformin should be added with a GLP-1RA, such as semaglutide or dulaglutide, for patients with atherosclerotic cardiovascular disease (ASCVD) or at high risk of CVD to improve their CVD outcomes, as long as there are no contraindications and regardless of whether patients meet the HbA1c target or not.
Once-weekly semaglutide is a novel long-acting GLP-1RA. Its breakthrough peptide structure can significantly extend the half-life to 7 days, making it suitable for once-weekly dosing. The global phase III clinical trial program Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) showed that the glucose-lowering effect of once-weekly semaglutide was significantly better than that of all the other comparators, and it had multiple cardiovascular and metabolic benefits, such as improving blood pressure, blood lipids, and body weight control [8,9,10,11,12,13,14,15,16]. The SUSTAIN China study showed that, with once-weekly semaglutide, the proportion of patients reaching the HbA1c target (HbA1c < 7%) in the Chinese population was as high as 86.1% [17]. Network meta-analyses between GLP-1RAs also found that once-weekly semaglutide had more favorable clinical benefits than other GLP-1RAs [18]. SUSTAIN 7 trial is a 40-week, multicenter, randomized, open-label, parallel-controlled clinical trial to compare the efficacy and safety of once-weekly semaglutide and dulaglutide in patients with T2D who remained poorly controlled on metformin monotherapy. Prespecified statistical analyses indicated that reductions from baseline in HbA1c and body weight were superior with semaglutide in both the high-dose comparison (semaglutide 1.0 mg versus dulaglutide 1.5 mg) and the low-dose comparison (semaglutide 0.5 mg versus dulaglutide 0.75 mg). A post-hoc analysis of SUSTAIN 7 analyzing the effects of once-weekly semaglutide 0.5 mg and dulaglutide 1.5 mg revealed that once-weekly semaglutide 0.5 mg led to similar improvement in glycemic control and more significant weight loss [19].
In addition to clinical benefits, the economic evaluation of novel interventions with appropriate comparators is important for healthcare decisions and clinical practice. In China, once-weekly semaglutide was included in the National Reimbursement Drug List (NRDL) through price negotiation in 2021 and has been eligible for reimbursement from 1 January 2022. However, the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide in China is unknown. Therefore, the objective of the current study was to assess the long-term cost-effectiveness of once-weekly semaglutide 0.5 mg and 1.0 mg versus dulaglutide 1.5 mg for the treatment of patients with T2D uncontrolled on metformin in China from the perspective of healthcare systems. Considering that the dose of dulaglutide 0.75 mg is rarely used in clinical practice in China, we included only dulaglutide 1.5 mg in this study.
Methods
Modeling Approach
This study was performed using the Swedish Institute of Health Economics Diabetes Cohort Model (IHE-DCM) (version 4.4.2) to evaluate the long-term health and economic outcomes of once-weekly semaglutide and dulaglutide in patients with T2D who were not controlled with metformin in China.
The IHE-DCM is a published, validated, and expert-reviewed model. In recent years, the IHE-DCM has been used in Sweden, Canada, and other countries for the economic evaluation of hypoglycemic drugs, including liraglutide, once-weekly semaglutide, oral semaglutide, insulin degludec plus liraglutide (IDegLira), and lixisenatide [20,21,22,23]. The IHE-DCM is built on the basis of Visual Basic for Applications (VBA) in Microsoft Excel. It can simulate the occurrence of diabetes-related complications by constructing Markov submodels. The IHE-DCM can simultaneously compare an intervention with 12 control groups. The cycle length is 1 year, and the maximum time horizon is 40 years. The model is highly flexible as most of the model parameters are defined by the user on the input sheet. The mortality risk equations are sourced from the United Kingdom Prospective Diabetes Study (UKPDS) 68 [24] or UKPDS 82 [25]. The macrovascular risk equations are sourced from UKPDS 68 [24], UKPDS 82 [25], the Swedish National Diabetes Register (NDR) [26], or the Australian Fremantle Diabetes Study (FDS) [27]. There is only one set of microvascular risk equations, which were sourced from other diabetes models [28,29,30].
The model projects the long-term outcomes of the populations on the basis of user inputs, including baseline cohort characteristics, risk factors of complications, clinical effects of treatment on biomarkers and drift of biomarkers, treatment cost, complication cost, and health utility values. To run probabilistically, user-specified standard errors could be entered next to each corresponding mean value input. All input parameters of the model are varied using a normal distribution, except for the event rates for hypoglycemia, which are varied using a log-normal distribution in order to avoid negative values. The model outputs include cumulative incidence of diabetes-related complications, life expectancy, quality-adjusted life years (QALYs), direct medical cost, incremental cost-effectiveness ratio (ICER), and cost-effectiveness scatterplots. We accessed to the model through a user agreement between the authors and the Swedish Institute for Health Economics to allow the authors to use the IHE-DCM.
Once-weekly semaglutide was compared with dulaglutide over a 40-year time horizon to capture the mortality due to diabetic-related complications and background mortality over lifetimes. Projected clinical and economic outcomes were discounted at a rate of 5.0% annually, according to the guideline of cost-effectiveness analysis in China [31]. To ensure transparency and reproducibility, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [32] and impact inventory suggested by Second Panel on Cost-Effectiveness in Health and Medicine [33] were completed during the establishment and reporting of this study and are included in the electronic supplementary material.
Baseline Population Characteristics
The baseline cohort characteristics were obtained from the head-to-head clinical trial SUSTAIN 7 (Table S1) [13]. The aim of SUSTAIN 7 was to observe the efficacy and safety of once-weekly semaglutide and dulaglutide in patients with T2D who remained poorly controlled on metformin monotherapy. A total of 1201 patients were included in the study. The mean age of patients was 55.67 years, the proportion of women was 44.79%, and the mean duration of diabetes was 7.42 years. A fixed dose-escalation procedure was used for once-weekly semaglutide groups. The dose was doubled every 4 weeks from a starting dose of 0.25 mg until the trial maintenance dose of 0.5 mg or 1.0 mg was reached. Dulaglutide 1.5 mg group received 1.5 mg without dose escalation throughout the study.
Treatment Effects
The clinical efficacy data of once-weekly semaglutide and dulaglutide were sourced mainly from the SUSTAIN 7 clinical trial [13]. At 40 weeks, the changes from baseline of biochemical parameters, including HbA1c, systolic blood pressure (SBP), total cholesterol (TC), body mass index (BMI), etc., were used to inform the treatment effects and rates of nonsevere and severe hypoglycemic events of once-weekly semaglutide and dulaglutide. The hazard ratio (HR) for cardiovascular events was derived from the cardiovascular outcome trials SUSTAIN 6 [34] and REWIND [35] to inform the cardiovascular protective effects of once-weekly semaglutide and dulaglutide, respectively (Table 1).
Treatment Switching and Long-Term Parameter Progression
In the base-case simulation, a simple treatment algorithm was assumed that patients received treatment with either once-weekly semaglutide (0.5 mg or 1.0 mg) or dulaglutide (1.5 mg) for 1 year and then switched to basal insulin. This was supported by data from real-world practice in China, which reported a mean duration of treatment with GLP-1 RA of 7 months [36]. The duration was rounded up to 1 year, as the treatment switch could occur only at the end of an annual cycle in the IHE-DCM. After 1 year when GLP-1RAs were discontinued, patients were assumed to intensify to basal insulin and continued this treatment for the remainder of their lifetime. In the simulation, the effects of once-weekly semaglutide and dulaglutide ceased after 1 year. The effect of basal insulin was applied from the second year using data of insulin glargine. The effect of insulin glargine on HbA1c, BMI, and hypoglycemic events among Chinese patients with T2D were derived from the study of Yu et al. [37].
After applying the treatment effects of once-weekly semaglutide and dulaglutide in the first year, HbA1c was assumed to drift upward at a rate of 0.14% annually throughout the whole study time according to data in the study of Kahn et al. [38]. Other biomarkers such as BMI, SBP, and lipid levels were assumed to remain at the same level after the treatment effects were applied. Risk equations in UKPDS 82 were used in the base-case analysis to predict the incidence of macrovascular disease and mortality.
Costs and Utilities
Cost in this study was estimated from a Chinese healthcare system perspective and inflated to 2021 Chinese Yuan (CNY) through the healthcare consumer price index (CPI) in China. Direct medical costs were measured, including pharmacy cost, cost of hypoglycemic events, and cost of treating micro- and macrovascular complications.
Drug costs were taken from the national average bidding price in January 2022. The use of diabetic medication resource was based on relative clinical trials. Costs of semaglutide were calculated according to the fixed dose-escalation procedure. Following intensification after 1 year, patients were assumed to receive 18.33 IU of basal insulin (insulin glargine), on the basis of a meta-analysis that evaluates the daily insulin dosage among Chinese patients with T2D [39]. Treatment adherence for each intervention was assumed to be 100%. Annual cost also captured concomitant medication (metformin) and needle use. The cost of self-monitoring of blood glucose (SMBG) was not captured since it is assumed that there was no difference in the frequency of SMBG between once-weekly semaglutide and dulaglutide. Annual pharmacy costs are presented in Table S2 in the electronic supplementary material.
Costs associated with diabetic complications and hypoglycemic events (nonsevere and severe) were obtained from published literature evaluating the cost of complications among Chinese patients with T2D [6, 40,41,42,43,44,45]. The utility data on diabetes-related health status were derived from published literature [20, 44, 46,47,48]. Diabetic complications cost at first year is a one-time cost, and cost in subsequent year was applied to all subsequent cycles. Utility reduction of complications was only applied to the cycle in which complications occur. Diabetic complications treatment costs and health state utilities applied in the analyses are presented in the electronic supplementary material (Tables S3 and S4).
Sensitivity Analyses
One-way sensitivity analyses were performed to evaluate the impact of individual parameters on the results of modeling. For probabilistic sensitivity analyses (PSAs), the nonparametric bootstrap technique was used to conduct 5000 sampling simulation calculations.
Ethics Statement
This article is based on previously conducted clinical trials and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base-Case Analysis
As shown in Table 2, in the 40-year simulation, the discounted life expectancy of patients in the once-weekly semaglutide 0.5 mg group and dulaglutide 1.5 mg group was 13.27 years and 13.23 years, respectively, and the discounted quality-adjusted life expectancy was 7.31 QALYs and 7.23 QALYs, respectively. Compared with the dulaglutide group, the discounted life expectancy and quality-adjusted life years of patients with once-weekly semaglutide 0.5 mg increased by 0.04 years and 0.08 QALYs, respectively. Similarly, compared with dulaglutide 1.5 mg, the discounted life expectancy and quality-adjusted life expectancy of the once-weekly semaglutide 1.0 mg group were associated with increases of 0.10 years and 0.19 QALYs, respectively. The health benefits of once-weekly semaglutide were mainly derived from reducing and delaying the occurrence and development of chronic complications of diabetes. The study showed that, compared with dulaglutide, treatment with once-weekly semaglutide reduced mortality and the cumulative incidence of various chronic complications (Table S5).
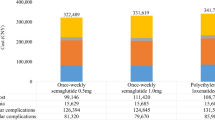
The discounted total direct medical cost of once-weekly semaglutide 0.5 mg and dulaglutide 1.5 mg was 301,684 CNY and 310,039 CNY, respectively. The cost saving of 8355 CNY for once-weekly semaglutide 0.5 mg mainly resulted from the reduction in the costs of pharmacy and complication treatment. Compared with dulaglutide 1.5 mg, the treatment cost of once-weekly semaglutide 0.5 mg decreased by 2163 CNY and the cost of treating complications decreased by 6087 CNY, respectively. The discounted total direct medical cost of once-weekly semaglutide 1.0 mg was 298,487 CNY, resulting in cost saving of 11,553 CNY compared with dulaglutide 1.5 mg. The cost saving came mainly from the reduction of the complication treatment cost, which completely offset the increase of the treatment cost (Fig. 1).
Sensitivity Analyses
Sensitivity analyses showed that the base-case results were robust to changes in the assumptions used and the input parameters (Table 3). In one-way sensitivity analyses in patients with inadequate control on metformin, once-weekly semaglutide 0.5 mg dominated dulaglutide 1.5 mg in all the analyses. Once-weekly semaglutide 1.0 mg dominated dulaglutide 1.5 mg in the majority of analyses and was cost-effective in the remaining three analyses (5-year time horizon 70,053 CNY per QALY gained; 10-year time horizon 20,831 CNY per QALY gained; HbA1c threshold of 7.0% for the treatment switching 42,072 CNY per QALY gained). When testing the progression of HbA1c (drift at a rate of 0.1% and 0.2%, using the UKPDS progression), SBP, and lipids, once-weekly semaglutide remained dominant in all four analyses. When using a baseline cohort characteristic of Chinese patients with T2D, once-weekly semaglutide was still a dominant treatment. In the test of applying difference sources of disutility for complications (heart failure, ischemic heart disease, lower-extremity amputation, end-stage renal disease, and stroke), the QALY gained of once-weekly semaglutide was similar to that of the base case. However, a significant increase in QALY gained was associated with once-weekly semaglutide when applying the BMI disutility of Lane et al.
Probabilistic sensitivity analyses showed that, at the willingness-to-pay threshold of one-time GDP per capita in China, the probabilities of once-weekly semaglutide 0.5 mg and 1.0 mg to be cost-effective compared with dulaglutide were both 100% (Fig. 2).
Discussion
Applying the IHE-DCM, this study found that once-weekly semaglutide 0.5 mg and 1.0 mg were both dominant (improving quality-adjusted life expectancy and reducing direct medical cost) treatments for Chinese patients with T2D whose glycemic levels were poorly controlled with metformin, versus dulaglutide 1.5 mg. This was the first long-term cost-effectiveness analysis of once-weekly semaglutide in the Chinese setting. These findings raised some subjects worthy of further discussion with references to international peer studies.
Regarding the clinical outcomes, this study projected that once-weekly semaglutide 0.5 mg and 1.0 mg were associated with increased quality-adjusted life expectancy of 0.08 QALYs and 0.19 QALYs, versus dulaglutide 1.5 mg. This finding was generally consistent with other long-term cost-effectiveness analyses of once-weekly semaglutide versus dulaglutide in other countries. For once-weekly semaglutide 0.5 mg versus dulaglutide 1.5 mg, it was estimated that once-weekly semaglutide was associated with improvement of 0.11 QALYs in Denmark [49], 0.04 QALYs in Slovakia [50], 0.04 QALYs in the UK [51], and 0.2 QALYs in Portugal [52]. In addition, for once-weekly semaglutide 1.0 mg versus dulaglutide 1.5 mg, once-weekly semaglutide was positively associated with improvement of 0.28 QALYs in Sweden [23], 0.34 QALYs in Denmark [49], 0.13 QALYs in the Netherlands [53], 0.05 QALYs in Canada [20], 0.07 QALYs in Slovakia [50], 0.10 QALYs in the UK [51], and 0.09 QALYs in Portugal [52]. All of these clinical outcomes supported the positive benefits of once-weekly semaglutide versus dulaglutide for patients with T2D in the long term. Consequently, our study suggested that once-weekly semaglutide 0.5 mg and 1.0 mg were dominant versus dulaglutide 1.5 mg in the long-term assessment within the Chinese healthcare systems, which was also found in the studies in Canada, Denmark, Slovakia, the UK, and the Netherlands [20, 49,50,51, 53]. While in Portugal, once-weekly semaglutide 0.5 mg and 1.0 mg were associated with the ICER of 7202€/QALY gained and 1490€/QALY gained, compared with dulaglutide 1.5 mg, respectively, and were considered a cost-effective option within the Portugal willing-to-pay threshold [52].
Two studies in Canada and Denmark also evaluated the cost-effectiveness of once-weekly semaglutide 0.5 mg compared with dulaglutide 0.75 mg. Their results showed that once-weekly semaglutide 0.5 mg was associated with improved quality-adjusted life expectancy and total cost saving, indicating that it was a dominant treatment [20, 53]. However, considering that dulaglutide 0.75 mg was rarely used in clinical practice in China, we did not include it in the analysis of this study.
The treatment of T2D usually focuses on reducing HbA1c, as glycemic control has been shown to reduce the incidence of diabetes-related complications in the long term [54,55,56,57]. More recently, the reduction of other risk factors, such as body weight and BMI, has shown further benefits [58, 59]. Once-weekly semaglutide was associated with greater reductions in body weight and BMI compared with dulaglutide. In our study, the disutility value of −0.006 per unit increase in BMI (> 25 kg/m2) was used in the base-case analysis, which was consistent with other studies [49, 51]. However, a recent study by Lane et al. [44] estimated the relationship between weight and quality of life in patients with T2D in Canada, and the results showed that, for each unit increase of BMI, there was an associated decrease in utility of 0.0472. This BMI disutility value has been used in a previous cost-effectiveness analysis of exenatide twice daily compared with insulin glargine once daily in Chinese patients with T2D [60]. In the sensitivity analysis of our study, when applying the disutility value of −0.0472 per unit increase in BMI, once-weekly semaglutide was associated with greater improvements in quality-adjusted life expectancy (+0.37 QALYs for semaglutide 0.5 mg, +0.82 QALYs for semaglutide 1.0 mg) compared with dulaglutide 1.5 mg.
Health utility data used in our study were obtained mainly from a systematic review that synthesized a range of utility values for 21 diabetes complications [46]. This systematic review provided a preferred set of disutility values for modeling complications associated with T2D, and reported the range of disutility value for each complication. The disutility values associated with diabetes complication were varied and may lead to considerable uncertainty of the results. Therefore, we performed a series of sensitivity analyses to test the uncertainty around these values, especially for the most frequently occurring micro- and macrovascular diabetes complications, including end-stage renal disease, lower-extremity amputation, heart failure, stroke, and ischemic heart disease. For these five complications, the preferred disutility values (−0.263, −0.280, −0.108, −0.164, and −0.090 for each complication, respectively) recommended by the systematic review were used in our base-case analysis, and the lowest value of each complication disutility value range (−0.053, −0.177, −0.051, −0.007, and −0.027, respectively) was used in the sensitivity analyses. Sensitivity analyses showed that the base-case results were robust to changes in the disutility values. However, it should be noted that most health utility data were not derived from the Chinese population since related data were lacking at present. It is very important to generate a set of utility values for diabetic complications based on the Chinese population to support cost-effectiveness modeling research in the future.
Unlike most previous studies, which assumed that patients received once-weekly semaglutide or dulaglutide for 3 years before initiation of basal insulin [50, 53, 61], our study assumed that patients receive once-weekly semaglutide or dulaglutide for 1 year, which was supported by data from real-world practice in China [36]. Sensitivity analyses using longer fixed duration (2 years and 3 years) of GLP-1 RA treatment suggested that once-weekly semaglutide was still a dominant treatment versus dulaglutide. Considering that, in real-world practice, a greater reduction in HbA1c would be associated with a delayed time to treatment intensification, treatment with once-weekly semaglutide is likely to result in a delayed time to intensification. When using an HbA1c threshold of 7.0% for treatment switching in the sensitivity analysis, once-weekly semaglutide 0.5 mg and 1.0 mg were associated with 1-year and 3-years delay in treatment intensification, respectively, compared with dulaglutide group (intensification at the third year for dulaglutide 1.5 mg, at the fourth and sixth year for semaglutide 0.5 mg and 1.0 mg). This resulted in more quality-adjusted life expectancy gained for once-weekly semaglutide 0.5 mg and 1.0 mg (+0.19 QALYs and +0.27 QALYs) but an increase in the total cost for once-weekly semaglutide 1.0 mg (+ 8891 CNY), compared with dulaglutide 1.5 mg. While there was no officially recognized willingness-to-pay threshold in China, at an estimated threshold of one-time GDP per capita (80,976 CNY/QALY gained), semaglutide 1.0 mg was still suggested to be a cost-effective treatment in this scenario.
As a chronic disease, diabetes and its complications have a lifelong impact on patients. To capture the lifetime health and economic outcomes of once-weekly semaglutide and dulaglutide in patients with T2D, a 40-year time horizon was used in the study. Sensitivity analysis over a time horizon of 30 years showed that once-weekly semaglutide 0.5 mg and 1.0 mg still dominated dulaglutide 1.5 mg. However, when the time horizon was reduced to 10 or 5 years, less QALYs gain and less cost saving associated with once-weekly semaglutide were obtained, perhaps because the benefits of once-weekly semaglutide require a longer time to be fully captured. When using the time horizons of 10 years and 5 years, once-weekly semaglutide 0.5 mg remained a dominant treatment compared with dulaglutide 1.5 mg, while semaglutide 1.0 mg was associated with slight increase in cost, with ICER values of CNY 20,831 per QALY gained and CNY 70,053 per QALY gained, respectively, suggesting that it was still a cost-effective treatment.
One advantage of this study was the inclusion of data from cardiovascular outcomes trials (CVOTs). Our previous systematic literature review of the cost-effectiveness of once-weekly semaglutide compared with other GLP-1RAs in T2D found that recent studies may have underestimated the cardiovascular benefits of once-weekly semaglutide [62]. In UKPDS 80, there were benefits in some macrovascular complications observed after many years, confirming the importance of HbA1c in these effects [63]. However, recent CVOTs have found the CV benefits after only a few years, indicating that CV complications may be influenced by aspects beyond the traditional risk factors such as HbA1c [64]. Once-weekly semaglutide has shown a 26% reduction in risk of major adverse CV events (MACE) in SUSTAIN 6 [34], and dulaglutide has been investigated in REWIND with a 12% reduction in risk of MACE [35]. Since there is no head-to-head CVOT between the two drugs, data derived from SUSTAIN 6 and REVWIND were directly applied in our study. It should be noted that the population in the two CVOTs were patients with T2D who have experienced CV or were at high risk of CV, which was different from our study. In addition, applying the CVOT data may lead to a double calculation of CV benefits. Considering the above limitations, a sensitivity analysis excluding cardioprotective effects of semaglutide and dulaglutide was performed. When setting the HR values of all CV events for semaglutide and dulaglutide as 1.0, once-weekly semaglutide 0.5 mg and 1.0 mg were still dominant compared with dulaglutide 1.5 mg. Another limitation of this study was using short-term data to project the long-term outcomes. Although this is one of the basic principles of health economics modeling, there are always some doubts concerning the accuracy of such an approach. To minimize the uncertainty, a published, validated, and expert-reviewed diabetes model was used, and extensive sensitivity analysis was conducted to test the robustness of the base-case findings. Moreover, the use of simulation models based on clinical assumptions and long-term risk equations to project long-term outcomes is widely recognized as a standard practice and is also recommended in guidelines for the economic evaluation of T2D interventions [65]. In fact, one purpose of health economic analysis, such as this study, is to provide information and to reduce the uncertainty of decision-making in the absence of long-term data.
In recent years, the IHE-DCM has been increasingly widely used in the economic evaluation of hypoglycemic drugs in Sweden, Canada, and other countries [20,21,22,23]. A study by Ericsson et al. [23] used IHE-DCM to evaluate the cost-effectiveness of once-weekly semaglutide versus dulaglutide and lixisenatide in patients with T2D in Sweden. Johansen et al. [20] assessed the cost-effectiveness of once-weekly semaglutide versus dulaglutide in the treatment of T2D in Canada. Recently, IHE-DCM has also been used in China to evaluate the cost-effectiveness of liraglutide compared with insulin glargine and exenatide [66, 67]. Furthermore, the IHE-DCM has been used for reimbursement decisions by the Dental and Pharmaceutical Benefits Agency (TLV) in Sweden, Canadian Agency for Drugs and Technologies in Health, and the National Health Security Administration in China. In addition, another important reason why IHE-DCM was used in this study was that it was available to authors through a user agreement between the authors and the Swedish Institute for Health Economics. However, the IHE-DCM has not been externally validated against the population of China, which was a major limitation of the study. To ensure the validity and reliability of study results, it is suggested that the pharmacoeconomics model of diabetes should carry out more external validation for different populations in the future studies.
Health economic evaluation is a technology based on a comprehensive analysis of economic and health outcomes, to evaluate and select the more cost-effective intervention. Pharmacy cost is one of the important factors affecting the long-term total cost and the pharmacoeconomic evaluation result. In fact, pharmacy cost may fluctuate greatly, especially when the health insurance list is changing dynamically. To better support decision-making, it is necessary to update the economic evaluation in time according to the updated pharmacy cost, which is also the proper way for the development and application of health economic evaluation.
Our study had some other limitations. Firstly, this study generalized international trial data to a Chinese population. However, SUSTAIN 7 is the only head-to-head clinical trial that evaluates the efficacy and safety of once-weekly semaglutide and dulaglutide. Secondly, treatment compliance is one of the key influence factors that should be considered in the analysis, but related setting has not been included in the IHE-DCM model yet. Therefore, treatment compliance was assumed to be 100% in this study, which may not be consistent with real-world practice. It is suggested that clinical data based on the Chinese population and real-world data that reflect clinical practice should be considered in future cost-effectiveness analyses of once-weekly semaglutide.
Conclusion
Compared with dulaglutide 1.5 mg, the use of once-weekly semaglutide 0.5 mg and once-weekly semaglutide 1.0 mg in Chinese patients with T2D with inadequate glycemic control by metformin therapy was projected to increase patients’ life expectancy and quality-adjusted life expectancy, and reduce total direct medical cost, implying its potential as a cost-effective intervention in the Chinese setting.
References
Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369: m997.
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chinese Journal of Diabetes Mellitus. 2021;13(04):315–409.
International Diabetes Federation. IDF Diabetes Atlas Brussels,Belgium2019 9th. https://diabetesatlas.org/atlas/ninth-edition/.
Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–23.
National Bureau of Statistics of China. China statistical yearbook (2020 edition) 2020. http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm.
He X, Zhang Y, Ruan Z, Li L, Wu J. The prevalence and related direct medical costs of chronic complications among patients with type 2 diabetes in China. Chin J Endocrinol Metabol. 2019;3(35):200–5.
Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(10):925.e11-22.
Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–60.
Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–54.
Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–66.
Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–66.
Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291–301.
Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–86.
Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(11):834–44.
Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–67.
Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100–9.
Ji L, Dong X, Li Y, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab. 2021;23(2):404–14.
Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving basal insulin. Diabetes Ther. 2018;9(3):1233–51.
Pratley R, Aroda V, Gondolf T, et al. 1000-P: Efficacy and safety of semaglutide 0.5 mg vs. dulaglutide 1.5 mg once weekly in type 2 diabetes: a post hoc analysis of SUSTAIN 7. Diabetes. 2019;68:1000-P.
Johansen P, Håkan-Bloch J, Liu AR, Bech PG, Persson S, Leiter LA. Cost effectiveness of once-weekly semaglutide versus once-weekly dulaglutide in the treatment of type 2 diabetes in Canada. Pharmacoecon Open. 2019;3(4):537–50.
Ericsson Å, Glah D, Lorenzi M, Jansen JP, Fridhammar A. Cost-effectiveness of liraglutide versus lixisenatide as add-on therapies to basal insulin in type 2 diabetes. PLoS ONE. 2018;13(2): e0191953.
Ericsson Å, Lundqvist A. Cost Effectiveness of insulin degludec plus liraglutide (IDegLira) in a fixed combination for uncontrolled type 2 diabetes mellitus in Sweden. Appl Health Econ Health Policy. 2017;15(2):237–48.
Ericsson Å, Fridhammar A. Cost-effectiveness of once-weekly semaglutide versus dulaglutide and lixisenatide in patients with type 2 diabetes with inadequate glycemic control in Sweden. J Med Econ. 2019;22(10):997–1005.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59.
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–33.
Ahmad Kiadaliri A, Gerdtham UG, Nilsson P, Eliasson B, Gudbjörnsdottir S, Carlsson KS. Towards renewed health economic simulation of type 2 diabetes: risk equations for first and second cardiovascular events from Swedish register data. PLoS ONE. 2013;8(5): e62650.
Davis WA, Knuiman MW, Davis TM. An Australian cardiovascular risk equation for type 2 diabetes: the Fremantle Diabetes Study. Intern Med J. 2010;40(4):286–92.
Bagust A, Hopkinson PK, Maier W, Currie CJ. An economic model of the long-term health care burden of Type II diabetes. Diabetologia. 2001;44(12):2140–55.
Brown JB, Russell A, Chan W, Pedula K, Aickin M. The global diabetes model: user friendly version 3.0. Diabetes Res Clin Pract. 2000;50 Suppl 3:S15–46.
Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–34.
Liu; GG. China guidelines for pharmacoeconomic evaluations. Beijing, China: China Market Press; 2020.
Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30.
Liu L, Zhang J, Zhang N, Zhen R, He X. POSC18 the real-world clinical effectiveness of GLP-1 receptor agonist liraglutide among patients with type 2 diabetes in China: based on existing healthcare data. Value Health. 2022;25(1, Supplement):S35–S6.
Yu M, Yuan GY, Zhang B, Wu HY, Lv XF. Efficacy and safety of dulaglutide by baseline HbA1c in Chinese patients with type 2 diabetes: a post hoc analysis. Diabetes Ther. 2020;11(5):1147–59.
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43.
Cai X, Yang W, Gao X, Zhou L, Han X, Ji L. Meta-analysis of the insulin dosage in Chinese type 2 diabetic patients receiving insulin treatment. Chin J Diabetes. 2016;24(6):490–507.
Xie X, Vondeling H. Cost-utility analysis of intensive blood glucose control with metformin versus usual care in overweight type 2 diabetes mellitus patients in Beijing, P.R. China. Value Health. 2008;11 Suppl 1:S23–32.
Su W, Li C, Zhang L, Lin Z, Tan J, Xuan J. Meta-analysis and cost-effectiveness analysis of insulin glargine 100 U/mL versus insulin degludec for the treatment of type 2 diabetes in China. Diabetes Ther. 2019;10(5):1969–84.
Duan X, Li Y, Liu Q, Liu L, Li C. Epidemiological characteristics, medical costs and healthcare resource utilization of diabetes-related complications among Chinese patients with type 2 diabetes mellitus. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):513–21.
Wu J, He X, Liu y. Cost-effectiveness analysis of insulin aspart 30 versus insulin glargine in patients with type 2 diabetes in China. Chin Pharm J. 2016;51(3):242–7.
Lane S, Levy AR, Mukherjee J, Sambrook J, Tildesley H. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin. 2014;30(7):1267–73.
Men P, Qu S, Luo W, Li C, Zhai S. Comparison of lixisenatide in combination with basal insulin vs other insulin regimens for the treatment of patients with type 2 diabetes inadequately controlled by basal insulin: systematic review, network meta-analysis and cost-effectiveness analysis. Diabetes Obes Metab. 2020;22(1):107–15.
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–9.
Pan CW, Sun HP, Zhou HJ, et al. Valuing health-related quality of life in type 2 diabetes patients in China. Med Decis Making. 2016;36(2):234–41.
Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide ER, liraglutide and lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther. 2019;10(4):1297–317.
Malkin SJP, Russel-Szymczyk M, Psota M, Hlavinkova L, Hunt B. The management of type 2 diabetes with once-weekly semaglutide versus dulaglutide: a long-term cost-effectiveness analysis in Slovakia. Adv Ther. 2019;36(8):2034–51.
Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–21.
Malkin SJ, Belbute DG, Hunt B, Hoxer CS, Martín V. PDB67 -Once weekly semaglutide versus dulaglutide for the treatment of patients with type 2 diabetes in Portugal: A long-term cost-effectiveness analysis based on SUSTAIN 7. Value Health. 2018;21:S129-S.
Hunt B, Malkin SJP, Moes RGJ, Huisman EL, Vandebrouck T, Wolffenbuttel BHR. Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res Care. 2019;7(1): e000705.
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30.
Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68(6):682–91.
Fruh SM. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):S3-s14.
Deng J, Gu S, Shao H, Dong H, Zou D, Shi L. Cost-effectiveness analysis of exenatide twice daily (BID) vs insulin glargine once daily (QD) as add-on therapy in Chinese patients with type 2 diabetes mellitus inadequately controlled by oral therapies. J Med Econ. 2015;18(11):974–89.
Gorgojo-Martínez JJ, Malkin SJP, Martín V, Hallén N, Hunt B. Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: once-weekly semaglutide versus empagliflozin. J Med Econ. 2020;23(2):193–203.
Ruan Z, Yang L, Shi H, et al. The cost-effectiveness of once-weekly semaglutide compared with other GLP-1 receptor agonists in type 2 diabetes: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2021;21(2):221–33.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Evans M, Johansen P, Vrazic H. Incorporating cardioprotective effects of once-weekly semaglutide in estimates of health benefits for patients with type 2 diabetes. Diabetes (New York, NY). 2018;67(Supplement_1).
American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–5.
Li H, Wang L, Yuan Z. PDB6 cost-effectiveness of liraglutide versus insulin glargine, BOTH in combination with metformin for patients with type 2 diabetes in China. Value Health Region Issues. 2020;22:S32–3.
Li H, Wang L, Yuan Z. PDB14 Cost-effectiveness of liraglutide versus exenatide, BOTH in combination with metformin for patients with type 2 diabetes in China. Value Health Region Issues. 2020;22:S34-S.
Acknowledgements
Funding
Funding for this study and the journal’s Rapid Service fee was provided by Novo Nordisk (China) Pharmaceuticals Co., Ltd, Beijing, China. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
The study was conceived and designed by all authors and conducted by Zhen Ruan and Yang Shen. Carolina Oi Lam Ung and Hao Hu drafted the manuscript, which was reviewed, revised, and approved by all authors.
Disclosures
Zhen Ruan, Carolina Oi Lam Ung, Weihao Wang, Jingyi Luo, Huimin Zou, Yan Xue, Yao Wang, Hao Hu, and Lixin Guo declare no conflict of interests. Yang Shen and Yawen Zhang are employees of Novo Nordisk.
Compliance with Ethics Guidelines
This article is based on previously conducted clinical trials and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and or analyzed during the current study are available from the corresponding author upon reasonable request. The health economic diabetes model described in this study is proprietary of the Swedish IHE, Lund, Sweden. Access to the model is at the discretion of the IHE.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ruan, Z., Ung, C.O.L., Shen, Y. et al. Long-Term Cost-Effectiveness Analysis of Once-Weekly Semaglutide versus Dulaglutide in Patients with Type 2 Diabetes with Inadequate Glycemic Control in China. Diabetes Ther 13, 1737–1753 (2022). https://doi.org/10.1007/s13300-022-01301-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01301-4