Abstract
Introduction
Imeglimin is a novel antidiabetic drug that amplifies glucose-stimulated insulin secretion (GSIS) and improves insulin sensitivity. Several randomized clinical studies have shown the efficacy of imeglimin for glycemic control in patients with type 2 diabetes (T2D). We aimed to evaluate the short-term effects and safety of imeglimin in terms of glycemic control, as assessed by intermittently scanned continuous glucose monitoring (isCGM).
Methods
This retrospective and observational study of 32 patients who were administered imeglimin in addition to existing treatment regimens was designed to evaluate glycemic profiles. The patients were monitored for more than 4 weeks, including the day of starting imeglimin. The changes in glycemic indices, including mean glucose level, coefficient of variation (CV), time in range (TIR) and time above range (TAR), before and after imeglimin administration were analyzed, and data on adverse effects were collected by interview.
Results
Imeglimin administration significantly improved the mean values of glucose (from 159.0 ± 27.5 mg/dL to 141.7 ± 22.1 mg/dL; p < 0.001), TIR (from 67.9 ± 17.0% to 79.5 ± 13.3%; p < 0.001) and TAR (from 29.4 ± 17.5% to 17.9 ± 13.7%; p < 0.001) and tended to improve CV (from 29.0 ± 6.1 to 27.4 ± 5.58; p = 0.058). The curves of 24-h mean glucose level for all 32 subjects were shifted downward from the baseline after imeglimin administration. The high mean glucose level, high TAR, low TIR, low body mass index and low C-peptide were related to the efficacy of imeglimin for glycemic control. The main adverse effects were gastrointestinal disorders, and the incidence of hypoglycemia was increased in cases receiving a combination of imeglimin plus insulin or a glinide agent.
Conclusion
Imeglimin clearly shifted the daily glucose profile into an appropriate range in Japanese T2D patients, indicating improvement of short-term glycemic control. Imeglimin is thought to be a promising therapeutic agent for T2D patients, especially those with a low insulin secretory capacity, which is a common phenotype in East-Asian subjects with glucose intolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There are limited data on the prescribing of imeglimin for patients with type 2 diabetes (T2D) in clinical practice. |
Evaluation of the short-term effects and safety of imeglimin in terms of glycemic control would provide important clinical information. |
What was learned from the study? |
Imeglimin clearly shifted the daily glucose profile into an appropriate range. |
The curves of 24-h mean glucose level were shifted downward from the baseline after imeglimin administration. |
Imeglimin is a promising therapeutic agent for T2D patients, especially those with a low insulin secretory capacity, which is a common phenotype in East Asians. |
Introduction
Type 2 diabetes (T2D) is caused by pancreatic β-cell dysfunction and insulin resistance resulting in the development of chronic hyperglycemia. To ameliorate poor glycemic control, several diabetic medications are currently applied in clinical practice, and new drugs are being developed which act through novel mechanisms to modify glucose metabolism. Imeglimin is a new oral antidiabetic drug and is the first member of a class of tetrahydrotriazine-containing compounds [1]. The main target of imeglimin is correction of mitochondrial dysfunction by modulating respiratory chain complex activities while decreasing reactive oxygen species production [2]. Imeglimin has been shown to amplify glucose-stimulated insulin secretion by improving the β-cell glucose response and to ameliorate insulin sensitivity in T2D patients [3]. A clinical trial showed that imeglimin treatment for 7 days clearly increased glucose-stimulated insulin secretion during hyperglycemic clamps in T2D patients [4]. On the other hand, imeglimin administration suppressed hepatic glucose production in high-fat-, high-sucrose-fed mice [5]. In addition, increased muscle glucose uptake was reportedly observed both in vitro and in vivo in rodents [6].
In a phase II dose-ranging trial conducted in Japanese subjects, an imeglimin dose of 1000 mg twice daily as monotherapy demonstrated optimal efficacy (− 0.94% HbA1c reduction vs. placebo) as well as favorable safety and tolerability [7]. Phase III trials also showed significant reductions in fasting blood glucose levels and HbA1c, − 0.72% from baseline, with imeglimin monotherapy [8]. While these clinical trial results apparently showed the strong potential of imeglimin as a novel treatment option for subjects with T2D, the therapeutic effects of imeglimin in clinical practice, such as daily glucose profiles and the risk of hypoglycemia, have yet to be investigated in detail.
Continuous glucose monitoring (CGM), which includes both real-time CGM (rtCGM) and intermittently scanned CGM (isCGM), has entered widespread use in recent years as a result of improvements in sensor accuracy and ease of application [9]. CGM allows for the direct observation of glycemic excursions and daily profiles, which can provide information that is beneficial when making immediate therapy decisions and/or initiating lifestyle modifications. CGM also provides the ability to assess glucose variability and identify hypo- and hyperglycemia patterns. New glucose control parameters are also emerging, such as glucose variability and time in range (TIR), adding valuable insights to those provided by traditional parameters [10].
The beneficial effects of imeglimin on glycemic control have already been demonstrated by phase III clinical trials. However, not enough is known about the actual changes obtained with imeglimin treatment in real-world settings. Therefore, this study aimed to evaluate the short-term effects and safety of imeglimin in terms of glycemic control in Japanese T2D patients, as assessed by isCGM.
Methods
Study Design and Participants
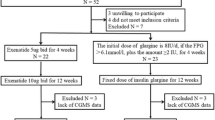
The subjects of this retrospective, observational study were recruited from among Japanese T2D outpatients visiting Iwate Medical University Hospital during the period from September through November 2021. The enrolled subjects were 20–75 years of age with HbA1c values ranging from 7.0 to 10%. Despite receiving various oral glucose-lowering agents or injectable therapy, none had yet achieved their glycemic control goal. We selected patients who were administered imeglimin (2000 mg/day) in addition to their existing treatment regimens in order to improve glycemic control. Their glucose values were monitored with an isCGM system (FreeStyle Libre®, Abbott Diabetes Care, Witney, UK) for more than 4 weeks, including the day of starting imeglimin. The isCGM dataset collected for approximately 2 weeks before starting imeglimin administration is referred to as the “before imeglimin” dataset, while that collected for approximately 2 weeks after starting administration is referred to as the “after imeglimin” dataset. Their diabetes medication regimens were otherwise unchanged at the time of starting imeglimin administration. The protocol for this retrospective observational study was approved by the Institutional Review Board of Iwate Medical University (approval number: MH2018-544-2021-435). The study was conducted according to the Declaration of Helsinki.
Glycemic Assessment
The patients’ glucose values were measured using the sensor-based FreeStyle Libre system readings obtained for more than 4 weeks. The sensor continuously estimated interstitial glucose levels and automatically stored glucose data every 15 min. The results from the isCGM were downloaded through web-based software and the proportions of time when glucose values were between 70 and 180 mg/dL (i.e., time in range; TIR), below 70 mg/dL (time below range; TBR), above 180 mg/dL (time above range; TAR) were calculated. The glucose level fluctuations during the day were determined as the coefficient of variation (CV). Additionally, the mean of daily difference (MODD) formula was applied to calculate the average difference between values obtained on different days but at the same time of day [11]. The incidence of severe hypoglycemia was defined as a glucose level of < 54 mg/dL. The clinical parameters, including HbA1c and glycoalbumin, were measured before initiating imeglimin administration, i.e., under casual conditions including fasting and after eating. The most recent serum C-peptide values were applied to analyses conducted before starting imeglimin. β-Cell function was assessed based on the C-peptide index (CPI), i.e., the ratio of casual serum C-peptide to blood glucose.
Statistical Analysis
Quantitative data are presented as median values (25th–75th percentile). Statistical analyses were conducted using the Wilcoxon signed-rank test and Spearman rank correlation analysis, as appropriate. The level of significance was set at p < 0.05. All statistical analyses were performed using SPSS version 26 (SPSS Japan Inc., Tokyo, Japan).
Results
Thirty-three patients were administered imeglimin, but one discontinued the medication due to severe nausea. Therefore, the results obtained from 32 patients were analyzed, and the clinical characteristics of these subjects are summarized in Table 1. The median HbA1c was 7.55 (7.03–8.02)% and BMI was 27.9 (23.8–30.0) kg/m2, indicating moderate obesity as compared to the general Japanese T2D population. Most of the patients were receiving concomitant treatment with other medications for diabetes, i.e., 12 were taking insulin and a glucagon-like peptide-1 receptor agonist (GLP-1RA) plus oral hypoglycemic agents (OHA), 3 insulin plus GLP-1RA, 6 insulin plus OHA, and 6 were taking a GLP-1RA plus OHA. In terms of the major medications of each type, 21 patients were receiving insulin, 21 GLP-1RAs, 15 sodium glucose transporter (SGLT)-2 inhibitors, 14 metformin, 8 dipeptidyl peptidase (DPP)-4 inhibitors and 8 glinides.
The period of isCGM examinations available for analyses was approximately 4 weeks overall: 14.1 ± 1.34 and 14.3 ± 1.09 days before and after administration, respectively. As shown in Table 2, imeglimin administration significantly improved mean glucose values from 161.0 (135.6–178.5) mg/dL to 141.0 (130.5–153.0) mg/dL (p < 0.001), and it tended to improve CV from 28.5 (24.9–31.2) to 26.7 (25.1–29.4) (p = 0.058) and MODD from 37.8 (26.4–49.7) mg/dL to 28.9 (23.0–41.8) mg/dL (p = 0.197). Similarly, apparent changes in indices derived from isCGM data were attributed to imeglimin administration: TIR changed from 69.5 (55.0–81.8)% to 82.0 (74.3–87.8)% (p < 0.001) and TAR changed from 28.5 (13.3–42.8)% to 17.0 (7.75–23.0)% (p < 0.001). By contrast, the incidence of hypoglycemia, as indicated by TBR, was unchanged after imeglimin administration. These changes in isCGM indices are shown in Fig. 1. The isCGM data showed that the curves of the 24-h mean glucose levels of the 32 subjects were shifted downward from the baseline after imeglimin administration (Fig. 2).
Changes in isCGM indices after imeglimin administration. The ratio of each of the glycemic indices evaluated by isCGM before (left bar) versus after (right bar) imeglimin administration in all 32 patients is shown. The colored bars show the average hours per day spent < 70 mg/dL (time below range; TBR: red), 70–180 mg/dL (time in range; TIR: green) and > 180 mg/dL (time above range; TAR: yellow)
Curves of mean glucose levels evaluated by isCGM before and after imeglimin administration. The 24-h glycemic variability based on the isCGM data for all 32 patients: the mean glucose level is shown for the periods both before and after imeglimin administration (both periods were about 14 days). The solid line indicates the glycemic variability before imeglimin administration; the dotted line indicates that after administration
Next, Spearman rank correlation analysis was performed to evaluate the baseline clinical parameters that contributed to the increased TIR following imeglimin administration. Change in TIR correlated positively with eGFR (r = 0.395), mean glucose evaluated by isCGM (r = 0.727) and TAR (r = 0.695), while it was negatively associated with BMI (r = − 0.378), CPI (r = − 0.555) and baseline TIR (r = − 0.610) (Table 3). The TIR increments were not associated with differences among the classes of diabetes medications combined with add-on imeglimin.
Adverse events related to imeglimin occurred in 12 patients; these were mainly gastrointestinal disorders: nausea in 4, abdominal discomfort in 2, constipation in 2, diarrhea in 1, and vomiting in 1 patient. As described above, 1 patient with particularly severe gastrointestinal disorders had to discontinue imeglimin. In addition, severe hypoglycemia (a glucose level of < 54 mg/dL) occurred in 7 patients, 5 of whom showed an increase in a glucose level of < 54 mg/dL after receiving imeglimin combined with insulin or glinides.
Discussion
This pilot study is, to our knowledge, the first to show the effects of imeglimin on short-term glycemic control evaluated by isCGM in Japanese T2D patients. Administration of imeglimin lowered mean glucose throughout the day and tended to improve glycemic variability without increasing hypoglycemia.
Several mechanisms underlying the glucose-lowering effects of imeglimin have been suggested by both in vitro and in vivo studies [12]. Improvement of β-cell function, especially glucose-stimulated insulin secretion (GSIS), was thought to be the main contributor to favorable diabetes control following imeglimin administration. Imeglimin increased the cellular nicotinamide adenine dinucleotide (NAD+) pool via the salvage pathway, resulting in amplified insulin secretion as well as the modulation of mitochondrial function with ATP generation in β-cells [13].
A previous study showed the efficacy of 7-day treatment with imeglimin at 1500 mg twice daily on GSIS, as assessed by hyperglycemic clamp in T2D patients [4]. Similarly, 1 week of imeglimin administration enhanced plasma insulin secretion in response to glucose loading in both chow-fed and high-fat-fed rats [14]. Relatively early onsets of pharmacological effects of imeglimin on insulin secretion were compatible with our isCGM results. Intriguingly, increased TIR with imeglimin administration was associated with low values of CPI, indicating a deterioration of β-cell function. In addition to direct effects enhancing GSIS, imeglimin reduced β-cell apoptosis by attenuating endoplasmic reticulum stress [15], leading to a modest increase in β-cell proliferation and the preservation of β-cell mass [16]. Our results suggest that the improvement of β-cell function by imeglimin treatment might be activated with a short-term medication regimen, especially in T2D patients with β-cell dysfunction.
As shown in Fig. 2, isCGM examination demonstrated a downward shift in the daily glucose profile following imeglimin administration, indicating that imeglimin reduced the whole-day glucose level—not only the level in the post-prandial period, but also that during the night. In previous rodent [14] and human hyperglycemic clamp studies [4], imeglimin-induced insulin secretion was observed not in the fasting state but after glucose loading. In contrast, a phase III trial showed a significant improvement in homeostasis model assessment (HOMA)-β values in patients receiving imeglimin treatment as compared to those given a placebo [8], suggesting improved insulin secretion while in the fasting state. The potential of imeglimin to enhance basal insulin secretion might have contributed to the observed whole-day glucose reduction.
Consistent with the aforementioned phase III trial [8], imeglimin treatment reduced fasting glucose levels in the present study. Imeglimin decreased hepatic glucose production, thereby efficiently contributing to lower nocturnal glucose levels, via a mechanism somewhat similar to that of metformin [17]. Moreover, imeglimin also enhanced insulin action in both the liver [6, 17] and muscle [5, 6]. In addition to basic research, clinical treatment with imeglimin for 24 weeks was reported to have significantly altered the Quantitative Insulin Sensitivity Check Index (QUICKI) values [8], an indicator of insulin sensitivity [18]. Suppression of hepatic glucose production as well as improved systematic insulin sensitivity were considered to play important roles in reducing fasting glucose levels, thereby contributing to the downward shift of daily glucose profiles.
Simultaneously, the downward shift of the glucose profile indicated relatively weak effects of imeglimin on postprandial glucose lowering. Small changes in postprandial glucose levels were supported by the statistical insignificance of CV in daily glucose values. These unexpected results might be partially explained by the relatively large proportion of our enrolled patients using GLP-1RA, because the phase III trial showed minimal efficacy in HbA1c reduction when imeglimin was used in combination with GLP-1RA [19]. Interestingly, the kinetics of the intracellular calcium ion increase in response to glucose in isolated islets from rats was delayed with imeglimin administration as compared to islets from those receiving GLP-1RA [13]. These electrophysiological assay results suggest that imeglimin-induced intracellular Ca++ mobilization occurs via an increase in the NAD+-cyclic ADP ribose pathway after GLP-1RA-induced intracellular Ca++ mobilization, in turn suggesting that the effect of imeglimin on GSIS might diminish with combination therapies including GLP-1RA. However, there was no difference in the glucose profile after imeglimin administration between the subgroups with versus those without GLP-1RA in our present observational study.
This study had several limitations. First, the observational and retrospective design was not sufficient to draw precise conclusions. Administration of a new drug might influence lifestyle modifications in patients with poor glycemic control. Second, the number of study subjects was small, reducing the statistical strength of associations. Third, the periods for casual serum C-peptide evaluation in response to imeglimin administration were variable. Since insulin secretory capacities might have changed during this period, the association between the effects of imeglimin and casual CPI could not be confirmed. Fourth, this study lacked controls, making only a before versus after comparison. Therefore, the glucose profile improvement following imeglimin administration might have been influenced by lifestyle modifications triggered by the add-on medication. Finally, the existing diabetes medications being taken by the enrolled subjects varied widely, suggesting that combinations of certain drugs with imeglimin likely influence glycemic control. Further prospective examinations employing a randomized design with a large number of subjects are required to clarify the associations between the glucose lowering effects of imeglimin and concomitant medications for diabetes.
Conclusion
Imeglimin clearly shifted daily glucose profiles into an appropriate range in Japanese T2D patients, indicating a short-term improvement in glycemic control. Imeglimin appears to be a promising therapeutic agent for T2D patients, especially those with a low insulin secretory capacity, which is a common phenotype in East Asians with glucose intolerance, and thus merits further study.
References
Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14:852–8.
Hallakou-Bozec S, Vial G, Kergoat M, et al. Mechanism of action of Imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021;23:664–73.
Doupis J, Baris N, Avramidis K. Imeglimin: a new promising and effective weapon in the treatment of type 2 diabetes. touchREV Endocrinol. 2021;17:88–91.
Pacini G, Mari A, Fouqueray P, Bolze S, Roden M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:541–5.
Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64:2254–64.
Fouqueray P, Leverve X, Fontaine E, et al. Imeglimin—a new oral anti-diabetic that targets the three key defects of type 2 diabetes. J Diabetes Metabol. 2011;2:1–8.
Dubourg J, Ueki K, Grouin JM, Fouqueray P. Efficacy and safety of imeglimin in Japanese patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled, dose-ranging phase 2b trial. Diabetes Obes Metab. 2021;23:800–10.
Dubourg J, Fouqueray P, Thang C, Grouin JM, Ueki K. Efficacy and safety of imeglimin monotherapy versus placebo in Japanese patients with type 2 diabetes (TIMES 1): a double-blind, randomized, placebo-controlled, parallel-group, multicenter phase 3 trial. Diabetes Care. 2021;44:952–9.
Ogawa W, Hirota Y, Osonoi T, et al. Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal-bolus insulin therapy: an open label, prospective, multicenter trial in Japan. J Diabetes Investig. 2021;12:82–90.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–603.
Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–8.
Konkwo C, Perry RJ. Imeglimin: current development and future potential in type 2 diabetes. Drugs. 2021;81:185–90.
Hallakou-Bozec S, Kergoat M, Fouqueray P, Bolze S, Moller DE. Imeglimin amplifies glucose-stimulated insulin release from diabetic islets via a distinct mechanism of action. PLoS ONE. 2021;16: e0241651.
Perry RJ, Cardone RL, Petersen MC, et al. Imeglimin lowers glucose primarily by amplifying glucose-stimulated insulin secretion in high-fat-fed rodents. Am J Physiol Endocrinol Metab. 2016;311:E461–70.
Li J, Inoue R, Togashi Y, et al. Imeglimin ameliorates β-cell apoptosis by modulating the endoplasmic reticulum homeostasis pathway. Diabetes. 2022;71:424–39.
Hallakou-Bozec S, Kergoat M, Moller DE, Bolze S. Imeglimin preserves islet β-cell mass in type 2 diabetic ZDF rats. Endocrinol Diabetes Metab. 2021;4: e00193.
Vial G, Lamarche F, Cottet-Rousselle C, Hallakou-Bozec S, Borel AL, Fontaine E. The mechanism by which imeglimin inhibits gluconeogenesis in rat liver cells. Endocrinol Diabetes Metab. 2021;4: e00211.
Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10.
Dubourg J, Fouqueray P, Quinslot D, Grouin JM, Kaku K. Long-term safety and efficacy of imeglimin as monotherapy or in combination with existing antidiabetic agents in Japanese patients with type 2 diabetes (TIMES 2): a 52-week, open-label, multicentre phase 3 trial. Diabetes Obes Metab. 2022;24:609–19.
Acknowledgements
We thank all study participants. We appreciate Ms. Makiko Asanuma and Ms. Asami Nozaki for their secretarial assistance.
Funding
This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research from AMED under grant number JP20am0101086 (support number 0338). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, have given their approval for this version to be published, and have agreed to be accountable for all aspects of the work.
Author Contributions
Authors’ contributions are as follows: T.O. recruited patients, collected the data and wrote the manuscript; M.S., K.N., and A.S., recruited patients and collected the data; N.T. conducted the statistical analyses; Y.H. reviewed and edited the manuscript; Y.I. managed the study design, contributed to relevant discussions, and reviewed the manuscript.
Disclosures
Yasushi Ishigaki has received lecture fees from Bayer Yakuhin, Kowa Pharmaceutical Company, MSD, Novartis, Novo Nordisk, Ono Pharmaceutical, Sanofi K.K., and Takeda Pharmaceutical; scholarship donations from MSD and Ono Pharmaceutical; and research funding from Daiichi Sankyo Company and Takeda Science Foundation.
Compliance with Ethics Guidelines
The study protocol was approved by the ethics review board of Iwate Medical University. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki 1964 and its later amendments, Good Pharmacoepidemiology Practices, and applicable local laws and regulations.
Data Availability
The datasets analyzed during the current study are not publicly available because they are sampled from medical data of Iwate Medical University but are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Oda, T., Satoh, M., Nagasawa, K. et al. The Effects of Imeglimin on the Daily Glycemic Profile Evaluated by Intermittently Scanned Continuous Glucose Monitoring: Retrospective, Single-Center, Observational Study. Diabetes Ther 13, 1635–1643 (2022). https://doi.org/10.1007/s13300-022-01298-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01298-w