Abstract
Heart failure is frequently associated with diabetes, and therapies which reduce mortality in people with heart failure and reduced ejection fraction (HFrEF) are often limited to drugs which modulate the renin–angiotensin–aldosterone system or heart rate control and occasionally to device therapy. Treatment is even more challenging in people with heart failure and preserved ejection fraction (HFpEF), with currently no approved therapy demonstrating a mortality-improving effect, limiting treatment to diuretics for the alleviation of the symptoms of fluid overload and risk factor management. Previous cardiovascular outcome trials for sodium-glucose co-transporter-2 (SGLT-2) inhibitors have demonstrated significant favourable outcomes for cardiovascular disease, heart failure hospitalisation and all-cause mortality. The aim of the nearly completed EMPEROR-preserved and EMPEROR-reduced trials is to determine the impact of empagliflozin on cardiovascular and heart failure outcomes in people with HFpEF or HFrEF with or without diabetes. The trials will add substantially to our understanding of SGLT-2 inhibitors in the treatment of HFrEF and may have major implications for the treatment of people with HFpEF. The study will also be powered to address the impact of empagliflozin on changes in renal function in people with and without diabetes and incident diabetes in the participants without diabetes at baseline. In this article we discuss the rationale for using SGLT-2 inhibitors in people with heart failure and explore the potential findings and importance of the ongoing EMPEROR-preserved and EMPEROR-reduced trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiovascular outcome trials have shown considerable improvement in heart failure hospitalisation and other cardiovascular outcomes in people using sodium-glucose co-transporter-2 (SGLT-2) inhibitors. |
There are currently no treatments demonstrating mortality benefit for people with heart failure and preserved ejection fraction (HFpEF). |
The EMPEROR trials will considerably add to our understanding of the future role for SGLT-2 inhibitors in people with heart failure, particularly HFpEF. |
Planned sub-analyses of the EMPEROR trials, which include analyses of changes in renal function and incident diabetes, will also be of particular interest. |
Introduction
The worldwide prevalence of heart failure is both substantial and increasing, with an estimated prevalence of more than 26 million people [1] and a substantial global economic burden estimated at US$108 billion [2]. Diabetes increases the risk of developing heart failure by more than twofold in men and fivefold in women, with an estimated 25% of people with diabetes suffering chronic heart failure [3]. Contrariwise, in one study 45.5% of people with heart failure and preserved ejection fraction (HFpEF) and 41.8% with heart failure and reduced ejection fraction (HFrEF) were diagnosed with diabetes, respectively [4]. People with diabetes and heart failure have poorer clinical outcomes, thought to be a consequence of greater cardiovascular risk factors and poor glycaemic control [5]. In people with diabetes, several mechanisms may explain the relatively high risk of HFpEF, including impaired cardiac metabolism and substrate utilisation, altered insulin signalling and cardiac deposition of advanced glycated end products [6]. Indeed, the CHARM programme investigators reported that diabetes was an independent predictor of morbidity and mortality in people with heart failure and that the relative risk of cardiovascular death or hospitalisation for heart failure (HHF) associated with diabetes was greater in participants with HFpEF than HFrEF [7]. Whilst pharmacological interventions improve outcomes in HFrEF, little evidence supports drug therapy for HFpEF [8]. As a result, superior treatments for heart failure, particularly in patients with HFpEF with underlying diabetes, are needed.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Cardiovascular Outcome Trials and Sodium-Glucose Co-Transporter-2 Inhibitors: Key Cardiovascular and Heart Failure Outcomes
A great deal has been learned about the non-glycaemic benefits associated with drug therapies for type 2 diabetes (T2D) since the undertaking of cardiovascular outcome trials (CVOTs). Indeed, CVOTs investigating sodium-glucose co-transporter-2 (SGLT-2) inhibitors have observed favourable cardiovascular outcomes associated with their use [9]. A recent meta-analysis observed that SGLT-2 inhibitor use in 38,723 participants from four CVOTs was associated with a significantly reduced rate of 3-point MACE (major adverse cardiovascular events) (hazard ratio [HR] 0.88), cardiovascular death (HR 0.88), HHF (HR 0.68) and all-cause mortality (HR 0.85) compared with placebo [10]. Similar findings had also been observed in a previous meta-analysis [11].
The first CVOT to report cardiovascular outcomes for SGLT-2 inhibitors was the EMPA-REG study investigating empagliflozin in people with T2D. The study investigators noted a significant reduction in 3-point composite MACE compared with placebo (10.5 vs. 12.1%) and significant relative risk reductions for cardiovascular death (38%), HHF (35%) and all-cause mortality (32%) [12]. Following the findings of the EMPA-REG study, canagliflozin was reported to demonstrate superiority for 3-point MACE (HR 0.86) in the CANVAS programme, with its use found to result in significant reductions in cardiovascular death (HR 0.87) and HHF (HR 0.67) [13]. Subsequently, similar observations were made in the CREDENCE trial, in which canagliflozin use was associated with a significantly reduced risk of cardiovascular death, myocardial infarction (MI) or stroke (HR 0.80) and HHF (HR 0.61) [14].
The DECLARE-TIMI 58 trial investigated the use of dapagliflozin and demonstrated a non-significant reduction in the rate of 3-point MACE (HR 0.93) and a significantly lower rate of HHF (HR 0.73) in people with T2D and previous or a high-risk of cardiovascular disease [15]. Subsequently, the DAPA-HF study explored the use of dapagliflozin in people with or without underlying T2D and a diagnosis of HFrEF. The study investigators observed a significant relative risk reduction versus placebo for cardiovascular death (9.6 vs. 11.5%) and worsening heart failure (10.0 vs. 13.7%) over 18 months in participants using dapagliflozin, with similar results in people with or without underlying diabetes [16]. The outcomes of the DAPA-HF study resulted in the US Food and Drug Aministration (FDA) recently approving dapagliflozin for use in people with HFrEF [17].
Most recently, the VERTIS-CV study investigating ertugliflozin use did not demonstrate superiority for 3-point MACE for cardiovascular death, nonfatal MI or stroke, although there was a significant reduction in the HHF rate compared with placebo (2.5 vs. 3.6%) [18]. In the absence of the forthcoming full publication and analysis of heart failure subtypes, one putative explanation for these different findings is variations in study populations between CVOTs. Indeed, 10% of the study population in the EMPA-REG trial had underlying heart failure [12] compared with 23.1% of participants in the VERTIS-CV study. Additionally, more than 80% of the participants in the VERTIS-CV study had HFpEF and just 19.4% had HFrEF [19], although the proportion of participants with HFpEF in the EMPA-REG study was not reported. Whilst the analysis is currently unpublished, it may be that the high proportion of participants with heart failure and HFpEF in the VERTIS-CV study may have affected the cardiovascular outcome results. Full publication of these results is awaited with great interest.
Established Heart Failure Therapies Improve Mortality in HFrEF but not HFpEF
Heart failure practice guidelines advocate that all patients with HFrEF should be prescribed angiotensin-converting enzyme (ACE) inhibitors and beta blockers in the absence of any contraindication. In those patients with a moderately to severely impaired left ventricular ejection fraction, mineralocorticoid receptor antagonists should be considered, to further improve prognosis [20,21,22,23]. Following initiation and dose up-titration of these medications, clinicians should consider the use of medications such as hydralazine, sacubitril/valsartan, nitrites and ivabradine or, in some cases, device therapy such as cardiac resynchronization therapy [20,21,22,23].
The use of ACE inhibitors reduces renin–angiotensin–aldosterone system (RAAS) activity by inhibiting the conversion of angiotensin I to angiotensin II, which reduces systemic vasoconstriction, cardiac and vascular hypertrophy and the adrenal release of aldosterone, thereby modulating sodium and water retention. Likewise, mineralocorticoid receptor antagonists impede the RAAS by directly inhibiting the effect of aldosterone in the kidney, reducing sodium and water retention and improving cardiac remodelling [24]. Thus, there is good rationale for RAAS inhibition in people with HFrEF, and evidence from trials consistently demonstrates improved clinical outcomes in these patients [25]. Additionally, overactivation of the sympathetic nervous system in people with HFrEF is responsible for systemic vasoconstriction, increased heart rate, increased left ventricular afterload, cardiac remodelling and fibrosis. Thus, beta blockers reduce the toxic effect of adrenergic excess by inhibiting the chronotropic and inotropic effect of adrenaline and noradrenaline on the heart, supporting a regression in myocardial mass and cardiac remodelling, again with favourable cardiovascular outcomes in people with HFrEF [26].
Unfortunately, little clinical trial evidence supporting RAAS inhibition [25] or beta-blockade [27] exists for people with HFpEF, often limiting treatment to risk factor management and diuresis to improve symptoms of fluid overload [20,21,22,23]. Despite current management approaches, the natural history of the disease is to progress, often with significant morbidity and mortality. Indeed, people with heart failure have a projected 5-year mortality rate of 75%, and 82% require hospital readmission [28]. Therefore, new drug therapies for people with HFpEF are essential, and the use of SGLT-2 inhibitors is attractive due to the significant number of patients with co-morbid diabetes.
Rationale for SGLT-2 Inhibitors in Heart Failure
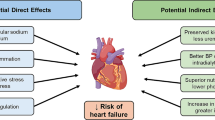
There is strong rationale for the use of SGLT-2 inhibitors in people with heart failure. These drugs inhibit the SGLT-2 protein in the proximal convoluted tubule of the nephron, reducing the reuptake of sodium and glucose to induce natriuresis and glucose-mediated osmotic diuresis, thereby improving glycaemia through enhanced urinary glucose excretion and improving blood pressure through urinary glucose and sodium excretion. However, the cardiovascular benefits of empagliflozin in the EMPA-REG study could not be explained by improved blood pressure, glycaemic control and lipids alone [29], implying other mechanisms must explain the cardiovascular benefit of this family of drugs. Indeed, several mechanisms have been speculated to explain the impact of SGLT-2 inhibitors in people with heart failure [30]:
-
1.
Reduced cardiac preload Increased diuresis due to urinary sodium and glucose excretion reduces plasma and interstitial fluid volumes that in turn reduce cardiac preload and support cardiac remodelling [31]. This reduction in plasma volume is not associated with increased sympathetic nervous activity [32].
-
2.
Shift to ketone metabolism SGLT-2 inhibitors cause a shift in body metabolism to produce ketones, a more energy-efficient substrate than fatty acids or glucose and thereby improve myocardial energy efficiency [33]. However, the ketone body levels generated from SGLT-2 inhibition are too modest to shift cardiac fuel metabolism, leading some to question whether other mechanisms better explain their impact in heart failure [34].
-
3.
Adaptive cellular reprogramming By promoting urinary calorie loss by glycosuria, SGLT-2 inhibitors induce a state of ‘fasting mimicry’, thereby activating the enzymes sirtuin 1 (SIRT1) and adenosine monophosphate–activated protein kinase (AMPK). These enzymes have antioxidant and anti-inflammatory effects which may improve cardiac function [35, 36].
-
4.
Inhibition of sodium-hydrogen exchange Heart failure is associated with increased activity of the cardiac Na–H exchanger, which is inhibited by the SGLT-2 inhibitor empaglilozin in rabbits and rats [37]. Inhibition of the Na–H exchanger may improve sensitivity to diuretics and endogenous natriuretic peptides, and reduce cardiac hypertrophy, fibrosis and remodelling [38]. Nevertheless, these findings are yet to be substantiated in humans.
-
5.
Improved endothelial function In the DEFENCE study investigating dapagliflozin, use in patients with inadequately controlled glycated haemoglobin demonstrated significant improvements in flow-mediated dilation of the brachial artery and biochemical measures of oxidative stress over 16 weeks [39]. However, improved glycaemic control and other classes of diabetes medication have also been associated with improved measures of endothelial function [40], suggesting that the relationship between improved endothelial function and SGLT-2 inhibitors may be indirect rather than direct.
-
6.
Reduced cardiac fibrosis In rats, SGLT-2 inhibitors have been observed to have anti-fibrotic effects by inhibiting collagen synthesis and myofibroblast differentiation which may have significant impact on cardiac remodelling and fibrosis [41]. Again, these findings have not yet been corroborated in human studies. Indeed, the REFORM study did not find any impact of dapagliflozin on left ventricular end-systolic volume or other parameters of left ventricular remodelling in people with T2D and HFrEF [42].
Study Overview: The Emperor-Reduced and Emperor-Preserved Trials
The EMPEROR-reduced and EMPEROR-preserved trials are phase III international, multicentre, randomized, double‐blind, parallel‐group, placebo‐controlled trials investigating the effect of empagliflozin 10 mg daily in addition to standard medical therapy in participants with either HFrEF or HFpEF, respectively, followed up over a median of approximately 20–24 months [43, 44]. The EMPEROR-reduced trial commenced in March 2017 and is due to complete in July 2020, with over 3700 participants enrolled [45]. The EMPEROR-preserved trial enrolled almost 6000 participants, starting in March 2017, and is due to finish in November 2020 [46]. The study design and key inclusion/exclusion criteria are presented in Fig. 1 and Table 1, respectively. For both trials, the primary outcome is time to first event of the combined risk for cardiovascular death or HHF. Prespecified secondary outcomes include HHF events, changes in estimated glomerular filtration rate (eGFR) from baseline, time to chronic dialysis, renal transplant or sustained reduction of eGFR, cardiovascular death, all-cause mortality, all-cause hospitalisation, time to onset of diabetes and changes in clinical summary score of the Kansas City Cardiomyopathy Questionnaire [43,44,45,46].
What will the Emperor Trials Add to Current Knowledge?
The EMPEROR-preserved trial is the first randomised placebo‐controlled trial to explore the impact of SGLT-2 inhibitors in the treatment of HFpEF [44]. Given the current lack of specific drug therapies for people with HFpEF, the results from this study may have a major impact on clinical practice for these patients. Current treatments are unfortunately limited to risk factor management and the use of diuretics to improve symptoms, and a poor quality of life, morbidity and mortality are associated with the disease [28]. The study has enrolled a substantial number of participants, with 5988 participants included and an expected follow-up of around 24 months which should give the study sufficient statistical power.
The EMPEROR-reduced trial will add to the DAPA-HF study to determine the impact of SGLT-2 inhibitors in the management of people with HFrEF [43]. The DAPA-HF study included 4744 participants followed up for a median 18.2 months [16], while the EMPEROR-reduced trial includes 3733 participants over about 20 months, making the trials comparable in terms of study design and size [43]. Importantly, the results from the latter study will provide information that will support decision-making by clinicians on the choice of SGLT-2 inhibitor for people with HFrEF, allowing them to make informed choices for these patients.
Of additional interest is the potential for sub-analyses of changes in eGFR associated with empagliflozin use in those with or without underlying diabetes. Combining the two trials would include eGFR data for almost 10,000 participants with an eGFR > 20 ml/min/1.73 m2, providing substantial insight into the impact of empagliflozin on eGFR for up to 24 months. We recently reviewed CVOTs reporting secondary renal outcomes and observed that use of SGLT-2 inhibitors reduced progression to macroalbuminuria and delayed the decline in eGFR associated with diabetes [47]. Renal co-morbidity frequently co-exists in people with heart failure as a result of chronic fluid overload and the association of many treatments for heart failure with acute kidney injury [48]. Treatments which reduce the decline in eGFR in this particularly at-risk population would be welcomed.
Additionally, sub-analysis of incident diabetes in participants without pre-existing diabetes associated with empagliflozin use will be possible. Indeed, in the DAPA-HF study 157 of the 2605 participants who did not have T2D at baseline developed diabetes. Of these, 93 of 1307 (7.1%) participants receiving placebo and 64 of 1298 (4.9%) participants using dapagliflozin developed diabetes over the trial period, representing a relative risk reduction of 32% in those receiving dapagliflozin [49]. Importantly, participants with prediabetes, higher body mass index and lower eGFR were more likely to develop diabetes, suggesting the potential for targeted diabetes prevention therapies with SGLT-2 inhibitors [49]. Again, comparison with the combined cohort of almost 10,000 participants across the two EMPEROR trials will be interesting and add significantly to the findings from the DAPA-HF study.
Conclusions
The EMPEROR-preserved and EMPEROR-reduced trials will provide novel insight into the use of empagliflozin for people with HFpEF or HFrEF. Additionally, they will improve our understanding of the impact of SGLT-2 inhibitors on changes in renal function and the use of SGLT-2 inhibitors to prevent diabetes. Therefore, the results of these trials will add significantly to information on this subject gained from previous trials and hopefully provide novel therapies for people with heart failure to improve current clinical outcomes which are all too often poor.
References
Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11.
Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–76.
Rosano GM, Vitale C, Seferovic P. Heart failure in patients with diabetes mellitus. Card Fail Rev. 2017;3(1):52–5.
Echouffo-Tcheugui JB, Xu H, DeVore AD, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get with The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20.
Wilkinson MJ, Zadourian A, Taub PR. Heart failure and diabetes mellitus: defining the problem and exploring the interrelationship. Am J Cardiol. 2019;124(S1):S3–S11.
Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol. 2018;34(5):632–43.
MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–85.
Bonsu KO, Arunmanakul P, Chaiyakunapruk N. Pharmacological treatments for heart failure with preserved ejection fraction—a systematic review and indirect comparison. Heart Fail Rev. 2018;23(2):147–56.
Ali A, Bain S, Hicks D, et al. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c-translating evidence into practice. Diabetes Ther. 2019;10(5):1595–622.
Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Eng J Med. 2015;373:2117–288.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Food and Drug Administration (FDA). FDA approves new treatment for a type of heart failure. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 10 July 2020.
American College of Cardiology. Evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial—VERTIS CV. 2020. https://www.acc.org/latest-in-cardiology/clinical-trials/2020/06/16/11/24/vertis. Accessed 10 July 2020
Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11–23.
National Institute for Health and Care Excellence (NICE). Chronic heart failure in adults: diagnosis and management. 2018. https://www.nice.org.uk/guidance/ng106/chapter/Recommendations#treating-heart-failure-with-reduced-ejection-fraction. Accessed 10 July 2020.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e161161.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239.
Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failuree – Web Addenda: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(27):2129–200.
George J, Struthers AD, Lang CC. Modulation of the renin–angiotensin–aldosterone system in heart failure. Curr Atheroscler Rep. 2014;16(4):403.
Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol. 2013;10(3):125–34.
Safi S, Korang SK, Nielsen EE, et al. Beta-blockers for heart failure. Cochrane Database Syst Rev. 2017;12:CD012897.
Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20(2):193–201.
Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86.
Fitchett D, McKnight J, Lee J, et al. Empagliflozin (EMPA) reduces heart failure irrespective of control of blood pressure (BP), low density lipoprotein cholesterol (LDL-C) and HbA1c. Diabetes. 2017;66(S1):A312–A313313.
Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–17.
Dekkers CCJ, Sjöström CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(12):2667–733.
Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22.
Crawford PA. Refueling the failing heart: a case for sodium-glucose cotransporter 2 inhibition in cardiac energy homeostasis. JACC Basic Transl Sci. 2018;3(5):588–90.
Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43(3):508–11.
Kalra S, Jain A, Ved J, Unnikrishnan AG. Sodium-glucose cotransporter 2 inhibition and health benefits: the Robin Hood effect. Indian J Endocrinol Metab. 2016;20(5):725–9.
Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–73.
Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–9.
Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84.
Lambadiari V, Pavlidis G, Kousathana F, et al. Effects of different antidiabetic medications on endothelial glycocalyx, myocardial function, and vascular function in type 2 diabetic patients: one year follow-up study. J Clin Med. 2019;8(7):983.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310.
Singh JSS, Mordi IR, Vickneson K, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43(6):1356–9.
Packer M, Butler J, Filippatos GS, et al. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019;21(10):1270–8.
Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019;21(10):1279–87.
ClinicalTrials.gov. EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure with Reduced Ejection Fraction (EMPEROR-Reduced). 2020. https://clinicaltrials.gov/ct2/show/NCT03057977. Accessed 10 July 2020
ClinicalTrials.gov. EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure with Preserved Ejection Fraction (EMPEROR-Preserved). 2020b. https://clinicaltrials.gov/ct2/show/NCT03057951. Accessed 10 July 2020
Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11(2):369–86.
Clark AL, Kalra PR, Petrie MC, Mark PB, Tomlinson LA, Tomson CR. Change in renal function associated with drug treatment in heart failure: national guidance. Heart. 2019;105(12):904–10.
Inzucchi SE, Docherty K, Kober L, et al. 271-OR: ADA Presidents’ select abstract: effect of dapagliflozin on the incidence of diabetes: a prespecified exploratory analysis from DAPA-HF. Diabetes. 2020;69(S1):271-OR
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
David M. Williams has nothing to disclose. Marc Evans received financial support for consultancy from Novartis, Merck Sharp & Dohme Corp. and Novo Nordisk and has served on the speaker’s bureau for Novartis, Lilly, Boehringer lngelheim, Merck Sharp & Dohme Corp., Novo Nordisk, Janssen and Takeda. Marc Evans is also the Editor-in-Chief of Diabetes Therapy.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12646211.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, D.M., Evans, M. Are SGLT-2 Inhibitors the Future of Heart Failure Treatment? The EMPEROR-Preserved and EMPEROR-Reduced Trials. Diabetes Ther 11, 1925–1934 (2020). https://doi.org/10.1007/s13300-020-00889-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00889-9