Abstract
Introduction
The associations of chronic kidney disease (CKD) severity, cardiovascular disease (CVD), and insulin with the risks of major adverse cardiovascular events (MACE), mortality, and severe hypoglycemia in patients with type 2 diabetes (T2D) at high cardiovascular (CV) risk are not known. This secondary, pooled analysis of data from the DEVOTE trial examined whether baseline glomerular filtration rate (GFR) categories were associated with a higher risk of these outcomes.
Methods
DEVOTE was a treat-to-target, double-blind trial involving 7637 patients with T2D at high CV risk who were randomized to once-daily treatment with either insulin degludec (degludec) or insulin glargine 100 units/mL (glargine U100). Patients with estimated GFR data at baseline (n = 7522) were analyzed following stratification into four GFR categories.
Results
The risks of MACE, CV death, and all-cause mortality increased with worsening baseline GFR category (P < 0.05), with a trend towards higher rates of severe hypoglycemia. Patients with prior CVD, CKD (estimated GFR < 60 mL/min/m2), or both were at higher risk of MACE, CV death, and all-cause mortality. Only CKD was associated with a higher rate of severe hypoglycemia, and the risk of MACE was higher in patients with CVD than in those with CKD (P = 0.0003). There were no significant interactions between randomized treatment and GFR category.
Conclusion
The risks of MACE, CV death, and all-cause mortality were higher with lower baseline GFR and with prior CVD, CKD, or both. The relative effects of degludec versus glargine U100 on outcomes were consistent across baseline GFR categories, suggesting that the lower rate of severe hypoglycemia associated with degludec use versus glargine U100 use was independent of baseline GFR category.
Funding
Novo Nordisk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is limited evidence regarding the associations of severity of chronic kidney disease (CKD) with the risks of various outcomes in patients with type 2 diabetes (T2D). |
Data are particularly limited for older individuals with multiple comorbidities, where the associations of coexisting CKD, established cardiovascular disease (CVD), and basal insulin with the risks of outcomes are not clear. |
Our secondary analysis from the DEVOTE cardiovascular outcomes trial examined whether baseline glomerular filtration rate (GFR) categories or a prior medical history of CKD, CVD, or both were associated with higher risks of the above outcomes. |
What was learned from the study? |
In the DEVOTE cohort of patients at high cardiovascular risk, worsening baseline GFR categories were associated with progressively higher risks of cardiovascular events and death, with a trend towards higher rates of severe hypoglycemia. There were no significant interactions between randomized treatment and GFR category, although consistently lower rates of severe hypoglycemia were observed with degludec compared with glargine U100, regardless of prior CVD or prior CKD (there was no significant difference for those without prior CVD). |
The relative effects of degludec and glargine U100 on cardiovascular outcomes were consistent across the baseline GFR categories, suggesting that the lower rates of severe hypoglycemia observed with degludec versus glargine U100 were independent of baseline GFR category (there were only significant differences for GFR categories G2 and G3). |
Considering that there was an observed association between baseline GFR category and risk of death regardless of randomized basal insulin assignment, a better understanding of this association and methods to mitigate the incremental risk associated with declining GFR remain important clinical objectives. |
Introduction
Cardiovascular disease (CVD) and chronic kidney disease (CKD) are highly prevalent comorbidities in patients with type 2 diabetes (T2D) [1, 2]. Both are associated with an increased risk of death [3,4,5,6].
Historically, cardiovascular outcomes trials (CVOTs) have excluded patients with CKD [7], while standard trials of antihyperglycemic therapies have typically enrolled cohorts of younger patients at a lower risk of cardiovascular events [8]. However, as a result of industry guidance, CVOTs for new antihyperglycemic therapies now include patients at high risk of cardiovascular events (including those with CVD or CKD) [9, 10]. Recently, results from a number of CVOTs (and secondary analyses) of patients with T2D have been published that demonstrate the potential for some antihyperglycemic therapies to improve cardiovascular and/or renal outcomes [11,12,13,14,15]. Some of these CVOTs have also compared the effects of treatment on cardiovascular outcomes in patients with T2D stratified by baseline estimated glomerular filtration rate (eGFR) [11, 14, 16].
Antihyperglycemic medications in patients with differing levels of renal function can affect treatment efficacy and safety; for instance, they can lead to an increased risk of hypoglycemia [11] and/or increased risks of cardiovascular events and mortality [17,18,19,20,21,22,23], resulting in product-label cautions, warnings, and contraindications [11, 24]. As patients with a lower glomerular filtration rate (GFR) tend to have a higher burden of CVD, a longer duration of T2D, and more commonly require insulin therapy [11, 14], there is a need to better understand the relationships between CKD and CVD risk factors, outcomes, and the use of medications in this population (particularly those with severe CKD who have been excluded from most trials) [11].
DEVOTE, a CVOT comparing insulin degludec (degludec) and insulin glargine 100 units/mL (glargine U100) in a population at high cardiovascular risk and renal risk, presents an opportunity to evaluate cardiovascular outcomes in insulin-treated patients with T2D along with CVD and/or CKD [25]. Thus, the present analyses evaluated the associations of baseline GFR category, prior CVD, and/or prior CKD with the risks of major adverse cardiovascular events (MACE), cardiovascular death, all-cause mortality, and severe hypoglycemia. They also examined whether randomized treatment had an impact on these outcomes stratified by baseline GFR category.
Methods
Trial Design
The secondary analyses described below utilized data from the DEVOTE CVOT. Detailed descriptions of the protocol, methods, and primary results have been published previously [25, 26]. In brief, DEVOTE was a multicenter, prospective, treat-to-target, randomized, double-blind, active comparator CVOT that was designed to continue until at least 633 MACE (as confirmed by a central, blinded Event Adjudication Committee, EAC) had accrued. DEVOTE is registered with ClinicalTrials.gov (NCT01959529) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [27, 28]. The protocol was approved by an independent ethics committee or institutional review board for each center; written informed consent was obtained from each patient before any trial-related activities were performed.
Patients and Treatments
Patients eligible for inclusion in DEVOTE were those who had T2D treated with at least one oral or injectable antihyperglycemic agent and had glycated hemoglobin (HbA1c) levels ≥ 7.0% (53 mmol/mol) or < 7.0% and treated with ≥ 20 units/day of basal insulin. In the present analyses comparing patients by baseline renal function, only patients with eGFR data at baseline/time of randomization were included. In DEVOTE, patients were randomized 1:1 to receive either degludec or glargine U100 (both in identical 100-U/mL, 10-mL vials) blinded, administered by subcutaneous injection once daily between the evening meal and bedtime, in addition to the standard of care. Patients were eligible if they had a medical history of either at least one coexisting cardiovascular or renal condition and were aged ≥ 50 years, or if they had at least one among a range of pre-specified cardiovascular risk factors (microalbuminuria or proteinuria; hypertension and left ventricular hypertrophy identified by electrocardiogram or imaging; or left ventricular systolic and diastolic dysfunction identified by imaging or ankle/brachial index < 0.9) and were aged ≥ 60 years [26]. All patients were allowed to continue their pre-trial antihyperglycemic therapy with the exception of basal and premix insulins, which were discontinued.
Outcomes
The main outcomes in the present analyses were as per the primary analysis in DEVOTE [26]. The primary composite MACE outcome was time to first occurrence of EAC-confirmed cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included time to EAC-confirmed all-cause mortality and the number of EAC-confirmed severe hypoglycemic events. Severe hypoglycemia was defined in accordance with the American Diabetes Association criteria as an episode requiring the assistance of another person to actively administer carbohydrate or glucagon or to take other corrective actions [29]. Other secondary outcomes included the HbA1c level achieved, the fasting plasma glucose (FPG), and the self-measured blood glucose (SMBG).
Statistical Analysis
In a set of pre-planned analyses, patients were grouped by baseline GFR category according to their baseline renal function as defined by the Kidney Disease: Improving Global Outcomes guidelines [30]:
-
GFR category G1 (≥ 90 mL/min/1.73 m2, normal function)
-
GFR category G2 (60– < 90 ml/min/1.73 m2, mild impairment)
-
GFR category G3 (30– < 60 mL/min/1.73 m2, moderate impairment)
-
GFR category G4–5 (< 30 mL/min/1.73 m2, severe impairment).
Patients with a baseline GFR category of G4 (n = 207) or G5 (n = 7) were combined to ensure a sufficient number of patients and events in these categories were included for comparison with other categories. The reference group for comparison of outcomes was G1, as these patients were those with a normal baseline eGFR. Patient outcomes were also evaluated according to randomized treatment by baseline GFR category.
Comparisons between GFR categories, treatment differences within each GFR category, and the interaction between treatment and GFR category were investigated for all outcomes. Analyses of outcomes were all pre-specified except for the evaluation of the interaction between treatment and prior CKD (defined as eGFR < 60 mL/min/1.73 m2 at baseline) [30] and/or prior CVD at baseline (according to medical history) as well as the analyses of outcomes as a function of baseline eGFR (as a continuous measure). Analyses investigating the associations of prior CVD and/or prior CKD with outcomes utilized a pooled population (i.e., independent of the randomized treatment) of all patients with CVD (with and without CKD) or with CKD (with and without CVD). In addition, supplementary analyses compared the associations with outcomes in patients with prior CVD only to those with prior CKD only.
Time from randomization to first MACE, cardiovascular death, and all-cause mortality were analyzed using Cox proportional hazard regression models with treatment, baseline GFR category, and interaction between treatment and baseline GFR category as fixed factors. The number of severe hypoglycemic events was analyzed using a negative binomial regression model with log-link function and the logarithm of the observation time (100 patient years) as offset in addition to randomized treatment, baseline GFR category, and their interactions. Additionally, for all the endpoints mentioned above, the interaction effect was dropped from the corresponding models to obtain pooled contrasts for baseline GFR category. In the analysis of the cardiovascular component of the MACE outcome, any patient whose first event was death from noncardiovascular causes was censored at their time of death.
Sensitivity analyses of all the pre-specified analyses were conducted following adjustment for the following additional baseline covariates: sex, geographical region, age, diabetes duration, cardiovascular risk stratum, insulin use, and smoking status.
The relationship between baseline GFR and the proportion of events during 1 year was modeled by polynomials of up to five degrees, with the best model selected using Akaike’s information criterion [31].
Changes in HbA1c and FPG from baseline to 24 months by baseline GFR category were analyzed with a mixed-effects model for repeated measures (MMRM) within participants, using an unstructured residual covariance matrix among visits. Besides randomized treatment and baseline GFR category, the MMRM included a fixed effect for the baseline measure of the given outcome.
Interactions between visit and treatment, visit and GFR category, and visit and first dose were also included as fixed effects, as was the three-way interaction (treatment × GFR × visit). The pooled treatment population differences in change from baseline HbA1c and FPG were analyzed by baseline GFR category using the same model with interactions between visit and treatment, visit and GFR category, and visit and baseline included as fixed effects, but without the three-way interaction effect. Total insulin dose (U/kg) at 24 months was analyzed by GFR category using the same MMRM as above, but using an unstructured residual covariance matrix among visits at 3, 6, 9, 12, 15, 18, 21, and 24 months, with log(first dose) included as a fixed effect.
A two-sided P value of less than 0.05 was considered statistically significant, although it was not multiplicity adjusted (SAS version 9.4, SAS Institute, Cary, NC, USA). All statistical analyses by randomized treatment assignment followed the intention-to-treat principle.
Results
Patients
The median observation time in DEVOTE was 2.0 years for both treatments. In the pre-planned analyses from DEVOTE (n = 7637), data on baseline eGFR were available for 98% of patients (n = 7522). Similar to the overall DEVOTE cohort, this population consisted of approximately equal numbers of patients randomized to treatment with degludec (n = 3765) or glargine U100 (n = 3757). In the total population (n = 7637), established CVD (72%) was more common than CKD (38%) at baseline. Furthermore, the presence of both prior CVD and prior CKD (25%) was more common than the absence of both (15%).
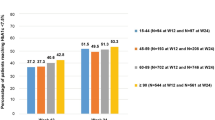
Comparing baseline characteristics and demographics across baseline GFR categories, patients with worse GFR categories were older, had a longer diabetes duration, included a lower proportion of males, had lower HbA1c levels, had higher body mass index (BMI) values, and were more likely to have established CVD/CKD (Table 1). Patients with worse GFR categories were also more likely to use insulin, angiotensin-receptor blockers, diuretics, and lipid-modifying drugs. In contrast, metformin and angiotensin-converting enzyme inhibitor use was more common in patients with less advanced GFR categories. Detailed comparisons of these data are provided in the supplementary results in the Electronic supplementary material (ESM), as are descriptions of baseline blood pressure and potassium levels.
Cardiovascular and Mortality Outcomes
Worse baseline GFR categories were associated with numerically higher risks of MACE, cardiovascular death, and all-cause mortality. Compared with patients with normal baseline eGFR (G1), risks were significantly higher for MACE (G3–5), cardiovascular death (G2–5), and all-cause mortality (G2–5) (Figs. 1, 2). Cardiovascular death accounted for 66% of all-cause mortality (n = 277/422), with similar contributions seen for all baseline GFR categories (63–69%). Cardiovascular death accounted for 41% of all MACE (n = 277/671); this increased from 27% in patients with GFR category G1 to 36, 50, and 53% in patients with GFR categories G2, G3, and G4–5, respectively. When evaluating outcomes using baseline eGFR as a continuous variable, the risks of MACE, all-cause mortality, and cardiovascular death were higher in patients with lower eGFR, but the relationship was nonlinear (Fig. S1 in the ESM).
Risks of various outcomes by baseline GFR category relative to GFR category G1. *Rate of events/100 patient years of exposure. GFR category G1 (solid circle) was used as the reference for GFR categories G2, G3, and G4–5 (open circles). Time from randomization to first MACE, cardiovascular death, and all-cause mortality were analyzed using Cox proportional hazard regression models with treatment, baseline GFR category, and interaction between treatment and baseline GFR category as fixed factors. The number of severe hypoglycemic events was analyzed using a negative binomial regression model with log-link function and the logarithm of the duration of observation time (100 patient years) as offset in addition to randomized treatment, baseline GFR category, and their interactions. Data on the proportion of patients with events and the rate of severe hypoglycemic events are based on observed numbers. CI confidence interval, CV cardiovascular, GFR glomerular filtration rate, MACE major adverse cardiovascular events
There were no significant interactions between randomized treatment (degludec versus glargine U100) and baseline GFR category for MACE, cardiovascular death, or all-cause mortality (Fig. 3).
Comparisons of associations of treatment with degludec or glargine U100 with the risks of MACE, cardiovascular death, and all-cause mortality and the rate of severe hypoglycemia by baseline GFR category. *P value for the interaction between treatment and baseline GFR category;†rate of events/100 patient years of exposure. Time from randomization to first MACE, cardiovascular death, and all-cause mortality were analyzed using Cox proportional hazard regression models with treatment, baseline GFR category, and interaction between treatment and baseline GFR category as fixed factors. The number of severe hypoglycemic events was analyzed using a negative binomial regression model with log-link function and the logarithm of the duration of observation time (100 patient years) as offset in addition to randomized treatment, baseline GFR category, and their interactions. Data on the proportion of patients with events and the rate of severe hypoglycemic events are based on observed numbers. The MACE and severe hypoglycemia analyses have already been published [25]. CI confidence interval, GFR glomerular filtration rate,glargine U100 insulin glargine 100 units/mL, MACE major adverse cardiovascular events
Severe Hypoglycemia
There was a nonsignificant trend towards higher rates of severe hypoglycemia with more advanced baseline GFR category (Figs. 1, 2), with the risk of severe hypoglycemia increasing with decreasing baseline eGFR (Fig. S1 in the ESM). There was no evidence of heterogeneity regarding the association between randomized treatment and baseline GFR category for severe hypoglycemia, as illustrated by the nonsignificant interaction (P = 0.9; Fig. 3).
Sensitivity Analyses of MACE, All-Cause Mortality, and Severe Hypoglycemia
The sensitivity analyses adjusting for baseline covariates were broadly consistent with the results of the primary analyses (Table S1 in the ESM). However, after adjustment, compared with patients with GFR category G1, patients with GFR category G2 no longer had significantly higher risks of cardiovascular death or all-cause mortality, and patients with GFR category G3 no longer had a significantly higher rate of severe hypoglycemia.
Associations of Prior CVD and Prior CKD with the Risks of MACE, Cardiovascular Death, All-Cause Mortality, and Severe Hypoglycemia
Patients with either prior CVD or prior CKD were at higher associated risk of MACE, cardiovascular death, and all-cause mortality than those without a history of either of these conditions (Fig. 4). In contrast, only prior CKD was associated with a significantly higher rate of severe hypoglycemia (Fig. 4).
Comparison of associations of prior CVD/CKD and no prior CVD/CKD with the risks of MACE, cardiovascular death, and all-cause mortality and the rate of severe hypoglycemia. *Rate of events/100 patient years of exposure. A more detailed exploration of the impact of a history of CVD or CKD on these outcomes is available in the ESM. Time from randomization to first MACE, cardiovascular death, and all-cause mortality were analyzed using Cox proportional hazard regression models with treatment, baseline GFR category, and interaction between treatment and baseline GFR category as fixed factors. The number of severe hypoglycemic events was analyzed using a negative binomial regression model with log-link function and the logarithm of the duration of observation time (100 patient years) as offset in addition to randomized treatment, baseline GFR category, and their interactions. Data on the proportion of patients with events and the rate of severe hypoglycemic events are based on observed numbers. CI confidence interval, CVD cardiovascular disease,CKD chronic kidney disease, glargine U100 insulin glargine 100 units/mL, MACE major adverse cardiovascular events
Upon comparing outcomes in patients with CVD only to those with CKD only, prior CVD was found to be associated with a higher risk of MACE than prior CKD (P = 0.0003), but there were no significant differences between patients with prior CVD and patients with prior CKD in the risks of cardiovascular death (P = 0.6), all-cause mortality (P = 0.2), and severe hypoglycemia (P = 0.1; Fig. S2 in the ESM).
A significantly higher risk of each outcome was consistently observed in patients with both prior CVD and prior CKD as compared with patients with neither of these conditions or with prior CVD alone (MACE, cardiovascular death, all-cause mortality: allP < 0.0001; severe hypoglycemia: P = 0.04 and P = 0.002, respectively; Fig. S2 in the ESM).
There were no significant interactions between randomized treatment and the association of prior CVD or prior CKD with MACE (P = 0.6 and 0.8, respectively), cardiovascular death (P = 0.2 and 0.9, respectively), all-cause mortality (both P = 0.2), or severe hypoglycemia (P = 0.09 and 0.6, respectively; Fig. 5). In addition to an absence of significant interactions, there were also no significant associations between randomized treatment and prior CVD status or prior CKD groups for MACE, cardiovascular death, and all-cause mortality. Regardless of whether patients had prior CVD or prior CKD, the rate of severe hypoglycemia was consistently lower with degludec than with glargine U100 except in patients with no prior CVD (Fig. 5). A more detailed exploration of the associations between a history of CVD or CKD and these outcomes is provided in Figs. S2 and S3 of the ESM.
Comparison of the associations of prior CVD/CKD/no prior CVD/CKD and treatment with degludec/glargine U100 with the risks of MACE, cardiovascular death, all-cause mortality and the rate of severe hypoglycemia. *P value for interaction between treatment and baseline CVD and CKD status;†rate of events/100 patient years of exposure. Times from randomization to first MACE, cardiovascular death, and all-cause mortality were analyzed using Cox proportional hazard regression models with treatment, baseline GFR category, and interaction between treatment and baseline GFR category as fixed factors. The number of severe hypoglycemic events was analyzed using a negative binomial regression model with log-link function and the logarithm of the duration of observation time (100 patient years) as offset in addition to randomized treatment, baseline GFR category, and their interactions. Data on the proportion of patients with events and the rate of severe hypoglycemic events are based on observed numbers. CI confidence interval, CVD cardiovascular disease,CKD chronic kidney disease, glargine U100 insulin glargine 100 units/mL, MACE major adverse cardiovascular events
Efficacy Measures
In general, differences in SMBG and changes in HbA1c and FPG were absent between baseline GFR categories G2–G5, with the exception of a lower total insulin dose (U/kg) in patients with a more advanced baseline GFR category (G1: 0.85, 95% confidence interval [CI] 0.82–0.89; G2: 0.76, 95% CI 0.74–0.78; G3: 0.69, 95% CI 0.67–0.71; G4–5: 0.61, 95% CI 0.54–0.68; see the supplementary results and Fig. S4 in the ESM).
Discussion
These secondary analyses from DEVOTE demonstrate that patients with a more advanced baseline GFR category were at greater risk of MACE, cardiovascular death, and all-cause mortality as compared with those in the normal baseline GFR category. In addition, severe hypoglycemia tended to be more common, albeit not significantly so, in patients with a more advanced baseline GFR category.
Patients with either prior CVD or prior CKD had higher risks of MACE, cardiovascular death, and all-cause mortality than those without these conditions, while those with both had the highest risks of these outcomes compared with those without prior CVD and prior CKD or those with only prior CVD. Patients with prior CVD had a significantly higher risk of MACE compared with those with prior CKD, and only prior CKD was associated with a significantly higher rate of severe hypoglycemia. When patients with prior CVD only were compared to those with prior CKD only, the risks of cardiovascular death and all-cause mortality were similar in both groups, despite the presence of a stronger association with CKD than with CVD.
The similar effects of randomized treatment across the GFR categories, together with the lack of significant interactions of baseline GFR category with randomized treatment for MACE, cardiovascular death, or all-cause mortality suggest that the type of basal insulin treatment applied did not affect the associations between GFR severity and outcomes. That is, regardless of the baseline GFR category, the results are consistent with those from the primary DEVOTE analyses [25], where the risks of MACE, cardiovascular death, and all-cause mortality were not found to differ significantly between the degludec and glargine U100 groups. Likewise, the similar estimated reduction in the rate of severe hypoglycemia with degludec versus glargine U100 (rate ratios: 0.62–0.77; significant differences were only observed for GFR categories G2 and G3) and the absence of a significant interaction between baseline GFR categories and randomized treatment for severe hypoglycemia suggest that degludec use resulted in a lower rate of hypoglycemia (as indicated by the present analyses and the primary DEVOTE analyses [25]) independently of the baseline GFR. The higher risks of MACE and all-cause mortality in patients with more advanced baseline GFR categories were driven by higher rates of cardiovascular death, which accounted for 44% of MACE and 66% of all-cause mortality.
These results are consistent with previous findings that patients with a lower baseline GFR are at high risk of MACE [22], all-cause mortality [32], and cardiovascular death [33]. While many studies have not found a higher risk of all-cause mortality in patients without CKD versus those with CKD [18, 20,21,22], the present study found that even mild CKD (category G2) is associated with a higher risk (hazard ratio 1.57, 95% CI 1.11–2.22) of all-cause mortality compared with normal GFR. This finding might be explained by the high burden of CVD in this population (71.7%) and the persistently high risk of cardiovascular death, accounting for 63–69% of all deaths across baseline GFR categories G2–G5. Indeed, a prior study of the contributions of CVD and CKD to all-cause mortality in patients with T2D found that CVD had the strongest association with risk of all-cause mortality in patients with and without CKD [23]. However, results in the present study demonstrate that prior CVD and prior CKD were similarly associated with risk of all-cause mortality and, if anything, prior CKD had the strongest association.
Risk factors predisposing patients with CKD to a greater risk of cardiovascular death include age, diabetes prevalence, diabetes duration, and hypertension, along with other risk factors associated with the development of atherosclerotic vascular disease [34]. In the present study, patients with CKD (G3–5) had a higher prevalence of established CVD/CKD and associated risk factors (e.g., longer diabetes duration, older age, and higher BMI) [35] than patients without CKD (G1–2). Other differences in terms of baseline antihyperglycemic and cardiovascular medications may reflect the longer duration and more advanced state of diabetes in patients with CKD.
If any aspect of the observed association between baseline GFR and risk of all-cause mortality (as reported in the present and previous studies [17, 18, 20, 21, 23, 36]) are causally connected (in other words, not an epiphenomenon), efforts to identify and limit the progression of CKD need to be pushed to the forefront of clinical care. The treatment of patients with T2D and at high risk of developing CVD or CKD is complex. Glycemic control and blood pressure management may limit the progression of CKD, but the effects of intensive glycemic control on cardiovascular outcomes have been inconsistent [37,38,39,40]. While glycemic control and blockade of the renin–angiotensin–aldosterone system remain paramount in the treatment of patients with T2D and CKD [41], the potential for antihyperglycemic medications to have favorable effects on cardiovascular and renal outcomes are being investigated [42]. For example, canagliflozin [11, 43, 44], empagliflozin [12, 45], liraglutide [15, 46], albiglutide [47], semaglutide [13], dapagliflozin [48], and dulaglutide [49] all have favorable effects on cardiovascular outcomes, while some of these also slow the progression of CKD compared with placebo [50].
While there is relatively little evidence that basal insulins differ in their ability to reduce cardiovascular events or mortality, there is evidence that basal insulins differ regarding the associated risk of severe hypoglycemia [25, 51, 52]. In the present study, the lack of significant interactions between randomized treatment and baseline groups (CKD and prior CKD/CVD) indicate that, as was the case for the overall DEVOTE cohort [25], the rate of severe hypoglycemia was lower with degludec compared with glargine U100 regardless of the baseline GFR. This finding is reassuring for clinicians, given that previous studies have reported that patients with CKD are predisposed to a greater risk of these events [37] and are consequently also at a greater risk of death [53].
While the DEVOTE CVOT has provided an ideal platform for exploring associations between baseline GFR category and outcomes in a T2D cohort at high cardiovascular risk, these post hoc analyses do have limitations. These include the likelihood that the DEVOTE eligibility criteria may have resulted in a patient cohort that is unrepresentative of the wider population of patients with T2D and CVD. An example of this that is relevant to the present study was the exclusion of patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2 [G4–5]) at screening. Despite this, 214 had severe renal impairment at baseline, including seven patients with eGFR < 15 mL/min/1.73 m2. An additional limitation is the lack of data on microalbuminuria at follow-up; however, as illustrated by the baseline data, CKD with microalbuminuria/proteinuria was not common in this cohort (37% of those with eGFR from 30 to < 60 mL/min/1.73 m2). Indeed, while the pathogenic role of proteinuria in renal diseases is clear, nonalbuminuric pathways conducive to renal function loss are more prevalent in patients with T2D and are the leading cause of end-stage renal disease [54].
Conclusion
In this large cohort of patients with T2D at high cardiovascular risk, worsening baseline GFR categories were associated with higher risks of MACE, cardiovascular death, and all-cause mortality. Patients with prior CVD, CKD, or both were at higher risk of MACE, cardiovascular death, and all-cause mortality. Only CKD was associated with a higher rate of severe hypoglycemia, and the risk of MACE was higher in patients with only CVD than in those with only CKD. The nonsignificant interactions between randomized treatment and study outcomes were in agreement with the results from the overall DEVOTE CVOT analyses suggesting that the lower rate of severe hypoglycemia with degludec than with glargine U100 (in the overall trial) [25] were independent of the severity of baseline CKD. Considering the observed association between baseline GFR category and risk of all-cause mortality, regardless of randomized basal insulin assignment, efforts to better understand this association and methods to mitigate the incremental risk associated with declining GFR remain important clinical objectives.
References
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83.
Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diabetes Res Care. 2016;4:e000154.
de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care. 1999;22:756–61.
Meisinger C, Doring A, Lowel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27:1245–50.
Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391:2430–40.
Rawshani A, Rawshani A, Franzen S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–18.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:273–80.
Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care editors’ expert forum. Diabetes Care. 2018;41:14.
US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.fda.gov/media/71297/download. Accessed July 5, 2019.
European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf. Accessed July 5, 2019.
Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–50.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes and chronic kidney disease: results from the LEADER trial. Circulation. 2018;138:2908–18.
Mann JFE, Ørsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–48.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81.
Roderick PJ, Atkins RJ, Smeeth L, et al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis. 2009;53:950–60.
So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29:2046–52.
Bruno G, Merletti F, Bargero G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50:941–8.
Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21.
Penno G, Solini A, Bonora E, et al. Defining the contribution of chronic kidney disease to all-cause mortality in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study. Acta Diabetol. 2018;55:603–12.
Arnouts P, Bolignano D, Nistor I, et al. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284–300.
Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32.
Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events)—DEVOTE 1. Am Heart J. 2016;179:175–83.
International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: guideline for good clinical practice. J Postgrad Med. 2001;47:199–203.
World Medical Association. WMA Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–95.
Kidney Disease: Improving Global Outcomes. Summary of recommendation statements. Kidney Int Suppl. 2013;3:5–14.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–23.
Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95.
Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–11.
Afsar B, Turkmen K, Covic A, Kanbay M. An update on coronary artery disease and chronic kidney disease. Int J Nephrol. 2014;2014:767424.
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–70.
Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73.
Molitch ME, Adler AI, Flyvbjerg A, et al. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 2015;87:20–30.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Dhar GC. Intensive glycemic control: implications of the ACCORD, ADVANCE, and VADT trials for family physicians. Can Fam Physician. 2009;55:803–4.
Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298–307.
Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2017;10:88–9.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019:394:121–30.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019:394:131–8.
Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Dailey G, Strange P. Lower severe hypoglycemia risk: insulin glargine versus NPH insulin in type 2 diabetes. Am J Manag Care. 2008;14:25–30.
McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–901.
Bolignano D, Zoccali C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol Dial Transplant. 2017;32:2194–9.
Acknowledgements
We thank the trial investigators, trial staff, and trial participants for their participation, and M. V. Bolten Jagd (Novo Nordisk) for insights that assisted in the development of this article.
Funding
The trial, these secondary analyses and the Rapid Service Fee were funded by Novo Nordisk. The sponsor contributed to data collection and statistical analyses. Novo Nordisk was involved in obtaining the data and designing these secondary analyses, provided logistical support, and ran all the statistical analyses, the results of which were evaluated jointly by the authors and the sponsor. DEVOTE research activities were supported at numerous US centers by Clinical and Translational Science Awards from the National Institutes of Health’s National Center for Advancing Translational Science.
Medical Writing and Editorial Assistance
Sam Mason and Germanicus Hansa-Wilkinson of Watermeadow Medical provided medical writing and editorial support. This was sponsored by Novo Nordisk.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Data from this analysis have been presented at ADA 2018 and EASD 2018.
Disclosures
Aslam Amod has received personal fees related to advisory boards and lectures from Novo Nordisk, Sanofi (South Africa), AstraZeneca, Merck Sharp and Dohme Corp., Lilly South Africa, Boehringer Ingelheim, Merck Biopharma, and Novartis South Africa. John B. Buse’s contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen; he has received grant support from Novo Nordisk, Sanofi, and vTv Therapeutics. He is a consultant to Cirius Therapeutics Inc., CSL Behring, Neurimmune AG, and Pendulum Therapeutics. He holds stock options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health. He is supported by a grant from the National Institutes of Health (UL1TR002489). Darren K. McGuire has received personal fees from Boehringer Ingelheim, Janssen Research and Development LLC, Sanofi US, Merck Sharp and Dohme Corp., Eli Lilly USA, Novo Nordisk, GlaxoSmithKline, AstraZeneca, Lexicon Pharmaceuticals, Eisai, Pfizer, Metavant, Applied Therapeutics, and Esperion. Thomas R. Pieber has received research support from Novo Nordisk and AstraZeneca (paid directly to the Medical University of Graz) and personal fees as a consultant from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk, and Roche Diabetes Care. TRP is also the Chief Scientific Officer of CBmed (Center for Biomarker Research in Medicine), a public-funded biomarker research company. Rodica Pop-Busui has received research support (to the University of Michigan) from AstraZeneca, and is supported by grants from the National Institutes of Health (NIDDK-1-R01-DK-107956-01, UC4 DK101108). Payment for Richard E. Pratley’s services were directed to AdventHealth (formerly Florida Hospital), a non-profit organization; he also received consultancy and speaker fees from AstraZeneca, Takeda, and Novo Nordisk; consultancy fees from Boehringer Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical Co. Ltd., Janssen Scientific Affairs LLC, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Pfizer, and Eisai, Inc.; and research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals, Inc., Eli Lilly, Merck, Sanofi US LLC, and Takeda. Bernard Zinman has received grant support from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk and consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi. Ting Jia is a full-time employee of and holds stock in Novo Nordisk A/S. Marco Bo Hansen is a full-time employee of and holds stock in Novo Nordisk A/S. Thomas Mark is a full-time employee of and holds stock in Novo Nordisk A/S. Neil R. Poulter has received personal fees from Servier, Takeda, Novo Nordisk, and AstraZeneca in relation to speakers’ fees and advisory board activities (concerning diabetes mellitus); and research grants for his research group (relating to type 2 diabetes mellitus) from Diabetes UK, National Institute for Health Research Efficacy and Mechanism Evaluation (NIHR EME), Julius Clinical, and the British Heart Foundation.
Compliance with Ethics Guidelines
DEVOTE is registered with ClinicalTrials.gov (NCT01959529) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [27, 28]. The protocol was approved by an independent ethics committee or institutional review board for each center; written informed consent was obtained from each patient before any trial-related activities.
Data Availability
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9924881.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Amod, A., Buse, J.B., McGuire, D.K. et al. Glomerular Filtration Rate and Associated Risks of Cardiovascular Events, Mortality, and Severe Hypoglycemia in Patients with Type 2 Diabetes: Secondary Analysis (DEVOTE 11). Diabetes Ther 11, 53–70 (2020). https://doi.org/10.1007/s13300-019-00715-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00715-x