Abstract
Intensive human exploitation of the Antarctic fur seal (Arctocephalus gazella) in its primary population centre on sub-Antarctic South Georgia, as well as on other sub-Antarctic islands and parts of the South Shetland Islands, in the eighteenth and nineteenth centuries rapidly brought populations to the brink of extinction. The species has now recovered throughout its original distribution. Non-breeding and yearling seals, almost entirely males, from the South Georgia population now disperse in the summer months far more widely and in higher numbers than there is evidence for taking place in the pre-exploitation era. Large numbers now haul out in coastal terrestrial habitats in the South Orkney Islands and also along the north-east and west coast of the Antarctic Peninsula to at least Marguerite Bay. In these previously less- or non-visited areas, the seals cause levels of damage likely never to have been experienced previously to fragile terrestrial habitats through trampling and over-fertilisation, as well as eutrophication of sensitive freshwater ecosystems. This increased area of summer impact is likely to have further synergies with aspects of regional climate change, including reduction in extent and duration of sea ice permitting seals access farther south, and changes in krill abundance and distribution. The extent and conservation value of terrestrial habitats and biodiversity now threatened by fur seal distribution expansion, and the multiple anthropogenic factors acting in synergy both historically and to the present day, present a new and as yet unaddressed challenge to the agencies charged with ensuring the protection and conservation of Antarctica’s unique ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica hosts a complexity and antiquity of terrestrial biodiversity and biogeography that has remained widely unappreciated until the last decade. Terauds et al. (2012) and Terauds and Lee (2016) defined 16 distinct terrestrial Antarctic Conservation Biogeographic Regions (ACBRs) within the area of Antarctic Treaty governance. In parallel with this strong regionalisation of Antarctic biogeography, it is also now appreciated that much of the terrestrial biodiversity contained within the ACBRs is endemic at regional and even sub-regional scales, with ancient evolutionary origins and divergences within the continent (Convey et al. 2020). These regions, and their contained biodiversity, are therefore of high conservation value, and the ACBRs are recognised as key conservation tools by the Committee for Environmental Protection (CEP) of the Antarctic Treaty Consultative Meetings, the governance body of Antarctica.
Of the 16 ACBRs, five are located in the western coastal regions of the Antarctic Peninsula and the Scotia Arc, a region more generally known as the maritime Antarctic. The region’s terrestrial ecosystems, which are present on the ~ 1.4% of its area that is seasonally free of snow and ice, are well developed and are characterised by biological components (cryptogamic groups—bryophytes, lichens, algae—biological [cyanobacterial and microbial] soil crusts and small invertebrates) that are fragile and sensitive to both physical damage and pollution. The dominant vegetation comprises bryophytes (mosses and liverworts), which lack the roots of higher plants and are only loosely connected to the underlying substrate. This vegetation and the soil crusts are vulnerable to being damaged or dislodged, while the apparently more robust lichens, many of which are firmly attached to rock and stone surfaces, are also vulnerable to physical fragmentation, particularly when trampled in their commonly desiccated state. The two flowering plants native to Antarctica, the grass Deschampsia antarctica and cushion plant Colobanthus quitensis, have greater resilience to physical disturbance (in particular, D. antarctica) though both can still suffer from crushing, fragmentation and manuring.
In recent decades, vulnerable coastal Antarctic terrestrial and lacustrine communities have been severely damaged by the trampling impacts of rapidly increasing fur seal numbers in the region. In this paper, we (i) describe the damage caused by fur seals to terrestrial ecosystems, (ii) unpick the sequence of human activities that led to the increase in fur seal numbers and distribution, (iii) detail earlier policy and management interventions and (iv) briefly discuss the future challenge of protecting Antarctic terrestrial habitats, recognising the need to use caution when considering human intervention in complex ecological systems.
Fur seal and human impacts on Antarctic terrestrial ecosystems
While research has been undertaken on the impacts of human activities on Antarctic vegetation and soils (Tin et al. 2009; Tejedo et al. 2012), less is known about those of the Antarctic fur seal (Arctocephalus gazella) whose presence has recently and rapidly expanded in the region since the mid-1970s. One location where fur seal impacts have been observed and studied in some detail for several decades is Signy Island in the South Orkney Islands. Here, changes in lichen diversity have occurred as a result of the substantial increase in trampling by fur seals transiently occupying the island each summer; at local scale, these include both reduced abundance and losses of species sensitive to physical disturbance and/or excessive nutrient contamination and increases in species positively associated with nutrient enhancement (Favero-Longo et al. 2011). Considerable damage to and loss of previously extensive cryptogamic vegetation and also grass on large areas of relatively flat ground at lower altitudes easily accessible from the coast have also been documented on the island and in the neighbouring Antarctic Specially Protected Area (No. 110) of Lynch Island (Smith 1988a, 1990, 1996a, 1996b, 1997; Cannone et al. 2016, 2017) (Figs. 1, 2, 3) as well as at other locations along the Antarctic Peninsula (Smith 1996a, b).
Panoramic view of Signy Island (South Orkney Islands) from the east. With the exception of Observation Bluff (at the far left of the picture), previously extensive vegetation across virtually all of the low lying and accessible ground in the mid- and foreground rapidly suffered heavy damage (estimated as > 75% complete loss or severe damage by Smith (1988a, b)) from fur seal trampling and manuring, which commenced in the late 1970s. Photomontage prepared by A.P. Taylor and S. Adlard
Illustrations of the form of damage caused by Antarctic fur seals to regional terrestrial ecosystems. a Hauling out of a dense group of male seals leads to extensive trampling/crushing and fragmentation, as well as over-fertilisation from faeces and urine (brown colouration), of an accessible coastal terrace on Lynch Island, South Orkney Islands (photo: P. Convey, February 1990); b, c individual fur seals preferentially select even small areas of vegetated ground as resting sites, rapidly crushing and destroying the existing vegetation (photos: P. Convey, January 1991); d the boundary of a seal exclosure on Bird Island, South Georgia, illustrating the direct impact of trampling by the recovered fur seal population on native tussac grass vegetation that was able to develop in more accessible coastal locations following the near extinction of the seals in the exploitation era (photo: British Antarctic Survey)
Map of Signy Island indicating the extent of low altitude areas easily accessible from the coast to fur seals. Following Cannone et al.’s (2016, 2017) findings that most vegetation damage occurs below 40 m a.s.l., but is apparent up to 60 m a.s.l., areas within these altitudinal ranges are indicated by yellow and orange shading, highlighting the eastern lowland areas of the island shown in Fig. 1, along with Cummings Cove in the south-west, that have suffered most impacts. Infrastructure on the island currently consists of the summer-operating research station, which typically hosts 5–8 staff for four months each summer. The island is accessed on foot from the station, with primary marine bird research and monitoring sites around the Gourlay Peninsula and North Point. The area actively protected from seal impact by construction of a fence on Berntsen Point is also indicated by red diagonal shading
Most Antarctic terrestrial ecosystems are characterised by being highly nutrient-limited (Convey et al. 2014), while lakes are generally oligotrophic, with some being ultra-oligotrophic (Heywood 1967; Heywood et al. 1980; Butler 2000; Quayle and Convey 2006; Izaguirre et al. 2021). Wind-blown fertilisation by marine-derived nutrients can occur over distances of several hundred metres from vertebrate concentrations, such as penguin colonies and seal wallows, and has been shown to be a positive driver of Antarctic terrestrial biodiversity (Bokhorst et al. 2019). However, if this fertilisation is accompanied by the intense trampling and excessive manuring experienced within and very close to colonies or wallows, most terrestrial diversity is rapidly eradicated at this local scale, often being replaced by the terrestrial foliose alga Prasiola crispa. Similarly, the recent presence of large numbers and densities of fur seals on islands such as Signy Island has led to rapid eutrophication of more accessible lakes close to the coast, which the seals utilise (Ellis-Evans 1990; Hawes 1990; Butler 1999; Quayle and Convey 2006).
The fragility of these terrestrial ecosystems is a recognised conservation issue and challenge in Antarctica. This challenge is driven by the combination of multiple factors, including the small overall amount of ice-free ground available, the small proportion of that area that hosts vegetation development, the nature of that vegetation and the competition resulting from the increasing demand for suitable ice-free locations where human activity is concentrated (Tin et al. 2009; Hughes et al. 2016; Brookes et al. 2019). It has been estimated that as little as 1.34% of ice-free ground on the western Antarctic Peninsula is vegetated (Fretwell et al. 2011). This estimate does not include the South Shetland Islands and South Orkney Islands, which host some of the richest terrestrial communities in the maritime Antarctic (Smith 1988a, 1990; Øvstedal and Smith 2001; Ochyra et al. 2008). Furthermore, Hughes et al. (2016) highlight that formal protection of vegetated ecosystems within the Antarctic Specially Protected Area (ASPA) system is both extremely limited and very uneven, with a total of only c. 16 km2 of vegetation protected within ASPAs across the entire continent, of which half is contributed by a single ASPA (No. 126 Byers Peninsula, Livingston Island). The Antarctic Treaty System’s Committee for Environmental Protection (CEP) and the independent Scientific Committee on Antarctic Research (SCAR) have developed guidelines that clearly recognise the vulnerability of these ecosystems and the need for careful management and avoidance of human impact on them (SCAR 2018). Nevertheless, many instances of such human damage, which can remain apparent for decades, have been and continue to be reported both beyond and within ASPAs, with many more not formally recorded (Smith et al. 1994; Tin et al. 2009; Braun et al. 2012, 2014; Peter et al. 2013; Convey 2020; Finger et al. 2021).
The ‘footprint’ of human activities is particularly significant and concerning in the South Shetland Islands and north-west Antarctic Peninsula, where multiple nations operate logistic hubs, research stations and field facilities, and tourism operators use regular visitor sites. For instance, virtually all ice-free areas or significant headlands in the South Shetland archipelago host research stations, refuges, camp sites, field instrumentation, regular research sites, or have become well-established visitor sites. Several ASPAs in the South Shetland Islands include semi-permanent field camps, refuges or field sites of national operators which are routinely used in support of scientific research (e.g. ASPAs 126 Byers Peninsula, 112 Coppermine Peninsula, 133 Nelson Island, 151 Lions Rump) or are immediately adjacent to research stations and/or some of the most visited tourist sites (e.g. 140 Deception Island, 150 Ardley Island, 128 Western shore of Admiralty Bay). All of this activity leads to human impacts of varying intensity, including within protected areas.
The major elements of anthropogenic impact and environmental change facing Antarctica, in particular the Antarctic Peninsula, and its ecosystems are arguably well recognised (Lee et al. 2017; Convey and Peck 2019; Siegert et al. 2019). However, frustration continues to be expressed regarding the slow pace of response within the Antarctic Treaty System in a number of areas relevant to environmental protection. Examples include the development of a systematic, effective and representative protected area system (Shaw et al. 2014; Hughes et al. 2016; Coetzee et al. 2017) and the prevention of further and mitigating existing anthropogenic damage to ecosystems, especially those in the vicinity of concentrations of human activity (Peter et al. 2008, 2013; Tin et al. 2009; Hughes and Convey 2014; Convey 2020). Other challenging issues include controlling the further expansion of human influence and cumulative impact on the Antarctic environment, with the inexorable expansion of research and tourism activities to ever more remote parts of the continent (Hughes et al. 2011; Pertierra et al. 2017; Brooks et al. 2019; Leihy et al. 2020) and the expansion of existing, and construction of entirely new, research stations and logistic facilities, which have historically faced little limitation in practice. The aforementioned activities have largely commenced despite the Protocol on Environmental Protection’s mandated protocols of assessment of environmental impacts and consultation of Treaty Parties (Lyons 2009; Hemmings and Kriwoken 2010), in part as it is also the case that the outcomes of the consultation process are only advisory and the Treaty has no enforcement powers.
Southern Ocean marine resource exploitation
Large-scale human impacts in the Southern Ocean regions commenced within a few years of the discovery of the remote sub-Antarctic islands in the latter part of the eighteenth century, quickly followed by the South Shetland Islands in the early nineteenth century. These discoveries took place in a very different era to the present day, driven by imperialism, the search for new territory and for opportunities to exploit new sources of valuable resources. The Southern Ocean rapidly became a primary target of this exploitation throughout the nineteenth century, driven in particular by increasing demand for the pelts of fur seal species found in the Antarctic and sub-Antarctic (Arctocephalus gazella, A. tropicalis) and oil from elephant seals (Mirounga leonina), high value commodities at that time (Bertrand 1971; Headland 1984, 2018; Townrow 1988; Trathan and Reid 2009). Large populations of fur seals on the sub- and other peri-Antarctic islands, but particularly on South Georgia and the South Shetland Islands (Fig. 4), were the first to suffer uncontrolled overexploitation, almost being driven to extinction (Bonner 1968; Forcada and Staniland 2009; Paijmans et al. 2021; Krause et al. 2022). The sequence of rampant overexploitation was followed by the great whales, with the development of, initially, shore-based whaling stations on several peri-Antarctic islands in the early twentieth century, and later of the pelagic whaling industry (e.g. Basberg 2004; Hart 2006). The exploitation of elephant seals (Mirounga leonina) was generally less intense and ultimately led to one of the first examples of pre-emptive management (Laws 1994). After the destruction of whale populations and demise of the whaling industry by the mid-1960s, overexploitation of marine resources continued through fisheries, with populations of some fish species being reduced to the extent that even in 2022, 40 + years after the establishment of the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) in 1982, full recovery has not yet occurred (Agnew and Nichols 1996; Grant et al. 2021).
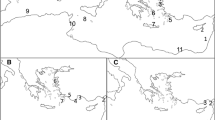
Map of the Southern Ocean around Antarctica showing the islands and island groups hosting breeding populations of Antarctic fur seals that were exploited around the northern Antarctic Peninsula, the Scotia Arc archipelagos and the sub-Antarctic islands. The primary population centre is South Georgia, hosting more than 95% of the global population of the species
Wider ecosystem impacts of marine overexploitation
One key consequence of this history of catastrophic overexploitation was its fundamental impact on the entire Southern Ocean marine ecosystem to the extent that, even now, its pre-exploitation structure and functioning remain unclear (Grant et al. 2021). Furthermore, it is not clear what trajectory ecosystem recovery will ultimately follow, even before the potential impacts of contemporary physical environmental change are considered (Griffiths et al. 2017; Convey and Peck 2019; Siegert et al. 2019; Morley et al. 2020; Chown et al. 2022; but see also Cavanagh et al. 2021). However, after large-scale disturbance, ecosystem theory suggests that recovery may lead to alternative stable states rather than simply a return to the original state (May et al. 1979; Bender et al. 1984; Mori 2011; Henderson et al. 2016; Yi and Jackson 2021).
The ramifications of this previous major human impact spread much farther beyond the Southern Ocean than is generally appreciated, with key impacts to the present day on other, non-marine, Antarctic ecosystems, which pose currently largely unaddressed conservation and management challenges. In their sub-Antarctic breeding and hunting grounds, both seal and whale exploitation had potentially considerable, but entirely unknown and undocumented, impacts on local terrestrial ecosystems at the time (see Convey and Lebouvier (2009) for discussion). With the size of pre-exploitation fur seal populations unknown, but thought to be of similar magnitude to those now existing after recovery (Forcada and Staniland 2009; Foley and Lynch 2020), and in the absence of significant native terrestrial vertebrates (Headland 1984; Convey 2017), the near extinction of fur seals would have greatly reduced their trampling and fertilisation of accessible terrestrial ecosystems. Aside from the concentrated impacts within dense breeding colonies, non- and post-breeding seals haul out in neighbouring coastal terrestrial habitats and can impact extensive areas hundreds of metres from the coastline, as well as to at least 60 m altitude, as illustrated in Fig. 2d (see Bonner 1985; Cannone et al. 2016). Such coastal areas host the highest development in terms of biomass and biodiversity of most Antarctic and sub-Antarctic native terrestrial vegetation and invertebrate communities. In South Georgia, where Antarctic fur seal populations have recovered and now represent more than 95% of the global population (Forcada and Staniland 2009), they have shown rapid expansion in the local occupied range over the last two to three decades around the coasts of the island (Payne 1977; see also discussion in Trathan et al. 2012) and this form of trampling damage is increasingly apparent both on South Georgia and at more southern locations (Smith 1988a, 1997; Favero-Longo et al. 2011; Cannone et al. 2016, 2017).
However, observations of recent trampling increase in the seals’ pre-exploitation breeding range on South Georgia also highlight that the contemporary perception of ‘typical’ sub-Antarctic coastal terrestrial habitats on this island may be inaccurate, with these now-impacted areas of habitat developing after the end of seal exploitation and persisting through most of the nineteenth and twentieth centuries. While detailed records of seal distribution on South Georgia are not available from this period, an assumption that the distribution of archaeological sealing relics (huts, caves, artifacts, etc.) reflects that of the seals being hunted is plausible. If so, then pre-exploitation seal distributions occupied much of the coastline of South Georgia and also parts of the South Shetland Islands (Headland 2009, 2014, 2018; Senatore 2019), although more physical evidence remains from elephant than fur sealing.
There must also have been major changes in transfer of marine-derived nutrients to land. This would have included short-term increases at a localised scale through the originally common practice of simply dumping carcass remains of both seals and whales on the shoreline where they were scavenged in part by birds such as giant petrels, skuas and sheathbills, thereby transferring nutrients to land in their guano. In the longer-term, decreases in nutrient transfer would also have taken place through the reduction of input of seal- and sometimes penguin-sourced faeces as their colonies, which are intense sources of aerosol dispersal of nutrients (cf. Bokhorst et al. 2019), were wiped out. Marine-derived nutrients are a major driver of terrestrial biodiversity in the sub-Antarctic and Antarctica (Smith 1988b; Zwolicki et al. 2015; Bokhorst et al. 2019). Thus, such changes in nutrient transfer must have had considerable, but completely undocumented, local impacts on terrestrial biodiversity.
The specific case of Antarctic fur seal population recovery
As noted, the South Georgian Antarctic fur seal population is thought to have recovered to a level comparable with or perhaps greater than its pre-exploitation level (e.g. Boyd 1993; Forcada and Staniland 2009; Foley and Lynch 2020). The much smaller South Shetland Islands population, the species’ southern-most breeding population, most likely recovered from a very small remnant after near extirpation, possibly reinforced by some migration from South Georgia (Reid et al. 2006; Krause et al. 2022). Recent detailed genetic studies support the two regional populations being distinct (Paijmans et al. 2021). Although the South Shetland Islands population appeared to have stabilised at a level of about one eighth of its pre-exploitation level by the early twenty-first century (Hucke-Gaete et al. 2004; Hoffman et al. 2018), it has subsequently shown a further substantial decrease in numbers suggested to be a consequence of a sustained increase in top-down predation of pups by leopard seals (Krause et al. 2022). However, a study of seal hair abundance in a core of accumulated terrestrial sediment on a coastal raised beach on King George Island (Sun et al. 2004) inferred large levels of variation in the size of the local fur seal population over the last 1500 years. The authors go on to speculate that this variation might relate to climatic factors and suggest that this population was subject to wide natural fluctuations even before the large-scale human destabilisation of the Southern Ocean ecosystem. The even smaller population on the remote and less accessible South Sandwich Islands received less impact from sealers and appeared to have only increased slightly between the only published surveys in the early 1960s and late 1990s (Holdgate and Baker 1979; Convey et al. 1999).
The overall recovery of fur seal populations has possibly been facilitated by the seals reaching sexual maturity much more rapidly than do baleen whales. The two overlap in their primary food source of krill, resulting in there potentially having been a ‘krill surplus’ available to support higher seal and penguin populations that was not available prior to whale overexploitation, although this has not been proven (Croxall 1992; Boyd et al. 1995; Trathan et al. 2012). With increasing evidence that at least some Antarctic whale populations are now recovering (Zerbini et al. 2019; McCormack et al. 2021), it is not clear whether the fur seal population ‘rebound’ will remain at its current level, or whether there will be some form of rebalancing with the increased number of whales. However, the consistent and large recent decreases in fur seal pup numbers and, by implication, adult populations on the South Shetland Islands (Krause et al. 2022) and South Georgia (Forcada and Hoffman 2014) over the last two decades, while having a number of possible contributing causes, may suggest this is happening already. Further important unknowns are the distribution and future trajectory of human exploitation of the krill resource, the primary food source exploited by fur seals and often described as one of the greatest remaining largely unexploited sources of protein on the planet (McBride et al. 2014; Trathan et al. 2021). Krill abundance and distribution are also intimately linked with the impacts of anthropogenic climate change on sea ice extent and distribution (Constable et al. 2014; Atkinson et al. 2019).
Interaction between fur seals and maritime Antarctic terrestrial ecosystems
An important feature of the Antarctic fur seal’s recovery is that their impact on terrestrial ecosystems now appears to extend to cover a much wider area of the maritime Antarctic, and involves considerably larger numbers of seals, than there is any evidence for having been the case pre-exploitation (Hodgson et al. 1998). There is no evidence that fur seals have occupied these areas and caused such damage previously, certainly since the end of the Pleistocene. The breeding range of this seal species has not changed, centred on South Georgia with far smaller outlying populations on the South Sandwich Islands, Bouvetøya and in parts of the South Shetland Islands as well as on other eastern sub-Antarctic islands (Table 1; Paijmans et al. 2021). The current impacts result from both immature males and post-breeding season bulls, but only very few females, from the South Georgia population dispersing widely to forage and haul out on land during the second half of the austral summer (Boyd et al. 1998; Waluda et al. 2010). They now come ashore in large numbers to rest and moult on the South Orkney Islands as well as on the islands off the north-east Antarctic Peninsula (James Ross Island area) and the length of the western Antarctic Peninsula as far south at least as Marguerite Bay (68–69° S). Conversely, tracking studies have shown that pelagic females and subadult and adult males from the South Shetland Islands population move south along the Antarctic Peninsula towards the end of summer and into the winter period (Arthur et al. 2017). Tracking studies of pelagic seals have not included reports or assessments of seals spending time ashore and it remains the case that there are no monitoring programmes regularly recording numbers of fur seals coming ashore at any location along the Antarctic Peninsula. The farthest south Antarctic fur seal records we are aware of are of single mature males observed on ice floes in the Ronne Entrance, south of Alexander Island (~ 73° S) on 12 February 2008 and in Lazarev Bay (69° 22′ S) on 17 February 2008 (Convey, pers. obs).
Long-term records of fur seal numbers in Antarctic locations are largely lacking; however, the South Orkney Islands may be the exception, with some degree of human presence and record keeping for much of the past 100 years or more. There are records of a very small number of fur seals being taken in the South Orkney Islands in the early nineteenth century (Marr 1935), but sealing records from the archipelago are very scarce, perhaps reflecting that it was historically more affected by sea ice extending up from the Weddell Sea. If so, fur seals may have been restricted from reaching the archipelago, as it seems unlikely that sealers would not have exploited any commercially viable population. Following the cessation of nineteenth century exploitation of fur seals on South Georgia and the South Shetland Islands, the first records of fur seals in the South Orkney Islands were from Laurie Island in 1936 (Headland 1989) and from Signy Island in 1948 (Laws 1973). The first thorough survey of the South Orkney Islands in 1971 (Laws 1981) recorded a total of 2035 fur seals. As noted by Hodgson et al. (1998), no reference to fur seal presence was made during the whaling period on Signy Island (1907/1908 to 1928/1929), although it seems likely that any seen would have been harvested. Annual numbers on Signy Island increased from a few dozen in the 1960s to a few hundred by the late 1970s, but then very rapidly increased to around 20,000 in the mid-1990s to early 2000s (maximum 21 303 in 1994; Waluda et al. 2010). Comparable increases in numbers were also reported from neighbouring Laurie Island (Vergani and Coria 1989; Carlini et al. 2006), while Yang et al. (2010) in a short terrestrial sediment study dating from the early twentieth century onwards inferred a similar pattern of increase in the local population on King George Island (South Shetland Islands). In the 1964/1965 summer, a small fur seal colony on Powell Island and neighbouring Michelsen Island in the South Orkney Islands contained around 550 adults and 35 pups, with a further smaller colony with pups on Fredriksen Island, and smaller numbers at Meier Point on Coronation Island and Monroe Island off the western point of Coronation Island (Smith pers. comm.). Laws (1981) reported the results of surveys of most of the accessible coastline of the South Orkney Islands that took place in 1971 and 1974. These surveys confirmed the presence of only three small breeding colonies (Gosling Islands, Monroe Island, Michelsen Island), with totals of 61 and 65 pups in the two years, respectively, and recorded only 12 male fur seals on Signy Island. Notably, the Signy Island numbers do not reflect the presence of a breeding population; the first pup was observed on the island in 1977 but, subsequently and even in the years with the highest counts, only a handful of females and even fewer pups have been recorded. Similarly, throughout the 1990s and 2000s, summer-dispersed male fur seals were reported from progressively more southern locations along the Antarctic Peninsula (e.g. Brabant Island, Furse (1986); Rothera Point, Marguerite Bay, Hughes (2003)), where they had not been reported previously in large numbers.
These observations initially led to a conclusion that no significant population of fur seals had been present on Signy Island or elsewhere in the South Orkney Islands since the retreat of ice after the Last Glacial Maximum (Smith 1988a, 1990). However, lake sediment studies on the island have refined this conclusion, confirming the presence of fur seal hairs in sediments dated up to c. 6500 years old (Hodgson et al. 1998; Hodgson and Johnston 1997) although, as with Sun et al.’s (2004) study on King George Island, with considerable variation over time. Importantly, Hodgson et al. (1998) suggested that the seal population size on Signy Island, as indicated by numbers of hairs retrieved throughout the sedimentary records, was consistently much smaller than that present from the 1980s onwards, concluding that this may be a clear indication of human interference in the Southern Ocean ecosystem. Unfortunately, although palaeolimnological studies have analysed sediment cores spanning the Antarctic Peninsula region from the South Shetland Islands, James, Ross Island, Hope Bay and south to Horseshoe Island, no studies appear to have searched for or reported fur seal hairs in these lake sediments. Similarly, seal hairs have not been reported amongst biological material recovered from studies of raised beaches, or from deep moss peat bank cores.
The role of climate change in southward fur seal distribution and haul-out site availability
There is no definitive explanation as to why the summer-dispersing male seals from South Georgia have expanded their previous range so dramatically. As noted above, a disturbed ecosystem will not automatically return to its original state after the disturbance, but this range expansion coincides with two particularly significant anthropogenically driven environmental changes in this region. First, the well-known Antarctic Peninsula regional air temperature warming in the second half of the twentieth century that was in large part driven by progressive reduction in extent and duration of winter sea ice west of the Peninsula and throughout the Scotia Arc. This meant that marine buffering of air temperatures over land played a much larger role than previously and, while warming took place in all seasons, the strongest trends were in the winter (Smith and Stammerjohn 2001; Stammerjohn et al. 2008; Turner et al. 2009; Eayrs et al. 2021). The retreat of sea ice may also have been accompanied by a parallel southward shift in distribution of Antarctic krill (Euphausia superba), the primary prey of fur seals and whose reproduction is linked with winter sea ice (Atkinson et al. 2019). Second, the warming resulted in ice and snow recession on land, both in terms of extent and earlier timing (Cook et al. 2005; Mulvaney et al. 2012), a process predicted to continue over the next century (Lee et al. 2017; Hughes et al. 2021). However, while ice recession will lead to increased area of habitat that can potentially be colonised by terrestrial biota, this habitat will primarily be formed through the expansion of coastal, low altitude areas that are already accessible to the summer-dispersing fur seals. Together, these two facets of regional environmental change give pelagic fur seals ease of movement and foraging farther south in open water and access to new areas of haul out on land. Interestingly, the previously strong Antarctic Peninsula warming trend paused and even reversed in the early years of the twenty-first century, with a series of colder years with increased snow and ice cover (Turner et al. 2009). This may be another factor leading to fur seal numbers on Signy Island subsequently remaining below the maxima reported in the mid-1990s (Waluda et al. 2010), and recent evidence of some recovery in previously heavily trampled areas of the two native flowering plants on the island (Cannone et al. 2022). Suggestively, Sancho et al. (2017) report similarly rapid, in this case negative, responses of South Shetland Islands lichens to this temporary cooler period. However, both studies serve to highlight how rapidly Antarctic terrestrial ecosystems can respond to different environmental drivers.
Fur seal policy and management
Early sealing regulation
By the start of the twentieth century, fur seals were considered all but extinct as a result of earlier overexploitation and no on-going sealing activity was commercially viable. The last commercial fur sealing expedition was to South Georgia in 1907, when 170 pelts were taken (Larson 1920). A British administration was established on South Georgia in 1906 and, while fur seal hunting was prohibited, permits for elephant sealing were granted from 1909 to the mid-1960s during which time c. 250 000 were taken (Hofman 2017). However, from the late 1950s, fur seal numbers on South Georgia had started to recover to a level where there was consideration of allowing renewed harvesting (Bonner 1958).
Convention for the Conservation of Antarctic Seals
In the early 1960s, exploratory research was undertaken to assess the viability of recommencing sealing in Antarctica (Øritsland 1970). Recognising concerns over the vulnerability of Antarctic seal species to commercial overexploitation and to reduce perturbations in the Antarctic marine ecosystem, in 1972, the Antarctic Treaty Consultative Parties developed the Convention for the Conservation of Antarctic Seals (CCAS) which entered into force in 1978 (available at: https://documents.ats.aq/keydocs/vol_1/vol1_13_CCAS_CCAS_e.pdf). CCAS prohibits the taking of Antarctic seals except in specific circumstances and in accordance with a permit. It established annual catch limits for each seal species, with taking of elephant and fur seals prohibited at any time, and established six sealing zones, a sealing season (1 September to the end of February) and three seal reserves. Contracting Parties are also required to provide annual reports on the sex, reproductive condition and age of seals taken. However, by the time CCAS entered into force in 1978, no sealing industry had developed in Antarctica, and the Convention was later largely superseded by the Protocol on Environmental Protection to the Antarctic Treaty (also known as the Environmental Protocol or Madrid Protocol; agreed in 1991, entered into force 1998) which, in effect, prohibited the commercial exploitation of seals (Annex II).
Specially Protected Species
Following the entry into force of the Antarctic Treaty in 1961, attention was directed towards conservation issues with the approval of the Agreed Measures for the Conservation of Antarctic Fauna and Flora (1964), which allowed for the addition of any native Antarctic species to the list of Specially Protected Species (SPS) following agreement by the Antarctic Treaty Consultative Meetings (ATCM). Through the Agreed Measures, Specially Protected Species (SPS) status was afforded to all species of the genus Arctocephalus (fur seal) within the Antarctic Treaty area (although only A. gazella is resident in the area), in response to the drastic population reductions resulting from earlier overexploitation. This high level of protection was continued when the Agreed Measures were used as the basis for the drafting of Annex II ‘Conservation of Fauna and Flora’ to the Environmental Protocol, despite the considerable increase in the fur seal population already documented during the intervening period. However, in 1999, the ATCM asked SCAR to provide a recommendation about the appropriateness of continued listing of fur seals as an SPS (Resolution 2, 1999). SCAR concluded that, on the basis of present populations and trends of these populations, fur seals could not be considered threatened or endangered under the IUCN Red List criteria and therefore were no longer in need of special protection (SCAR 2006). As a result, the ATCM removed fur seals from the list of SPS through Measure 4 (2006). The International Union on Nature Conservation defined the Antarctic fur seal as a species of least concern and not threatened in any part of its distribution in 2014 (Hofmeyr 2016). However, recent research has questioned this decision, on the basis that genetic studies have confirmed the existence of at least four genetically distinct sub-populations (South Shetland Islands, South Georgia, Bouvetøya, eastern sub-Antarctic islands) (Paijmans et al. 2021; Krause et al. 2022). As noted earlier, both the South Georgian and South Shetland Islands populations have consistently declined over the last two decades, and the decline in the latter has recently been described as potentially catastrophic, risking loss of an important component of the species’ genetic diversity (Krause and Hinke 2021; Krause et al. 2022).
Management of fur seal damage to terrestrial ecosystems
Where they are present on land in previously unoccupied areas during the austral summer, fur seals have major and long-term impacts on the fragile terrestrial vegetation, soils and microbial soil crusts that typify the maritime Antarctic (Block et al. 2009), both through direct trampling and excessive nutrient input from manuring (Fig. 2; Smith 1988a, 1997; Favero-Longo et al. 2011; Cannone et al. 2016, 2017). This often leads to complete destruction of the original communities, as documented for some of the previously best vegetated areas in the maritime Antarctic on Signy Island (Smith 1972, 1988a, 1997), although also encouraging the development of some nitrogen- or trampling-tolerant species such as the alga Prasiola crispa and ornithocoprophilous lichens (e.g. see Favero-Longo et al. 2011). In the sub-Antarctic, Haussmann et al. (2013) showed that trampling by the fur seal population on Marion Island can facilitate the local establishment of non-native vascular plants, while Frenot et al. (2001) reported that the non-native grass Poa annua had formed low grasslands around elephant seal wallows on sub-Antarctic Île de la Possession (Crozet Islands) and Kerguelen Island. Similarly, trampling and grazing by non-native reindeer have been suggested to have facilitated the wide distribution of P. annua in coastal valleys where whaling station activity was concentrated along the north-east coast of South Georgia. While the reindeer have recently been eradicated, the recovering fur seal population has now spread along this coast, providing continuation of the trampling activity. Such establishment events may also be possible at seal impacted sites in the maritime Antarctic, particularly considering the increasingly frequent reports of non-native plant introductions in the region (Hughes et al. 2015; Malfasi et al. 2019).
Fur seal impacts on ASPAs and ASMAs
Annex V to the Environmental Protocol allows for the designation of Antarctic Specially Protected Areas (ASPAs) to protect values including areas of outstanding or representative terrestrial ecosystems. However, designated protected areas in the Antarctic Peninsula and Scotia Arc region are not immune to the impacts of fur seal damage to their terrestrial ecosystems. Of the 25 coastal ASPAs within the Antarctic Peninsula, South Shetland Islands and South Orkney Islands, and potentially within the enhanced summer dispersal range of fur seals, the management plans of 20 (80%) mention the seals, and five (20%) confirm them to have caused damage. Vegetation within ASPA No. 113 Litchfield Island, Arthur Harbour, Anvers Island, Palmer Archipelago, and ASPAs in the South Orkney Islands, has been particularly affected by fur seal impacts (Shears and Richard 1994). All three Antarctic Specially Managed Area (ASMA) management plans on the Antarctic Peninsula mention fur seals. That of ASMA No. 7 Southwest Anvers Island and Palmer Basin explicitly identified an increase in numbers in the past 20 years, noting that fur seals had ‘destroyed many sites of rich flora in the region’ (Antarctic Treaty Secretariat 2019).
Practical management measures
Management activities to reduce seal impacts are potentially difficult and costly to put in place and generate conflict with regard to the protection of values associated with vegetation and terrestrial diversity compared to fur seal expansion. In essence, policymakers may have to establish whether it is more important to protect rare terrestrial communities (e.g. the unique communities developed on rare calcareous rocks on Signy Island) or fur seal populations that may have expanded following earlier human actions. However, it is the role of those environmental managers within national Antarctic programmes who must implement internationally agreed policy, to identify and put in place practical and affordable measures to provide the necessary protection. Annex II ‘Conservation of Antarctic fauna and flora’ to the Environmental Protocol states that ‘taking or harmful interference shall be prohibited, except in accordance with a permit’ (Article 3), with management of populations for conservation reasons not included in the list of reasons for provision of a permit.
Earlier and current attempts to limit fur seal access to vegetated areas through the use of fencing have had mixed success, with most being abandoned and removed due to the need for on-going repair following persistent seal damage. The extent of damage to terrestrial ecosystems on Signy Island, and also to the neighbouring small Lynch Island (ASPA No. 110 close to the south coast of Coronation Island, declared primarily to protect its exceptional lawns of the native grass Deschampsia antarctica), led initially during the 1980s to attempts at active management through the installation of fences across seal access routes to parts of the islands. On Lynch Island, this was achieved by fencing off two narrow gullies which were the primary access route. However, this was only partially successful, with only very infrequent maintenance possible and seals finding other routes to access the lawns, and the approach was abandoned around the time that Signy research station became a summer-only operating station in the mid-1990s with much reduced logistical support capability. On Signy Island, similar small fences were used in the 1980s and 1990s to attempt to prevent access to some lakes (e.g. Tranquil Lake, see Fig. 3) that were important to lake monitoring programmes operating on the island at that time (e.g. see Pearce et al. 2005). The only area of terrestrial habitat currently subject to protection attempts is the ‘Backslope’ (unofficial name), from close to the Signy research station in Factory Cove, up to Observation Bluff, which includes a significant area of well-developed and representative maritime Antarctic vegetation. A fence was first constructed to restrict seal access to this area in the early 1990s, with only partial success. The fence was replaced and strengthened in the 2010/11 summer season and remains in place to the present day (Figs. 3, 5a, b), being largely effective.
a The fence constructed on Signy Island to prevent fur seal access and protect one of the few remaining extensive areas of typical cryptogamic vegetation on the island (photo: M. Dunn). b Aerial view of the current Signy Station in Factory Cove, Signy Island, showing the position of the fence constructed across part of Berntsen Point to restrict Antarctic fur seal access to the richly vegetated ‘Backslope’ area leading up to Observation Bluff (see also Figs. 1, 3). While the research station area on Berntsen Point has been subject to human influence since the construction of a small whaling station in the early 1920s, the role of the fence in protecting the Backslope vegetation is clear (photo: A.P. Taylor and S. Adlard)
The installation of means of physical protection of terrestrial habitats from what might be regarded as native biota raises important points of principle in the debate as to how future conservation might be achieved in the Antarctic, particularly relating to the need for prioritisation of different competing factors or values (seals vs. terrestrial ecosystems). This is not a subject addressed within the text of the Environmental Protocol or in discussions to date within the Committee for Environmental Protection. It is important to consider the multiple aspects of direct and indirect human intervention that provide the foundations of the current situation (see overview provided in Table 2). These range from the original uncontrolled exploitation of multiple marine resources and destabilisation of the Southern Ocean marine ecosystem, through the different aspects of anthropogenic climate change that have more recently facilitated fur seal expansion southwards, to the further pressure placed on availability of terrestrial habitat area by expansion of human facilities, research sites and visitor locations.
Conclusions
With predicted warming trends continuing throughout the twenty-first century, as well as possible further expansion of summer-dispersing male seals to more southerly latitudes (e.g. to Pine Island Bay, 75° S, 102° W, or farther) where accessible ice-free terrain certainly exists supporting Adélie penguin colonies, consideration should be given to the potential for the fur seal breeding range to expand to suitable sites along the Antarctic Peninsula. The fur seal expansion, and its associated impacts, is clearly not simply a process driven by natural causes occurring within the native range of this seal species, and rather has a complex combination of very strong and originally anthropogenic drivers (Table 2). Furthermore, with increasing emphasis on the adoption of effective conservation and environmental protection practices since the Environmental Protocol entered into force in 1998, there is considerable effort and pressure to reduce and control sources of direct human impact such as trampling and vehicle damage in the Antarctic terrestrial environment. Given the strongly contrasting relative extent and scale of impacts of such human activities and the newly occupied fur seal range, the relative importance of contemporary direct human impacts on local environments and of those impacts—both direct and indirect—arising as consequences of previous interactions, will require further consideration by policymakers.
Examples, such as the foregoing, highlight the importance of considering all relevant factors when assessing the impacts of both distant and local human influences on Antarctic terrestrial and marine ecosystems. They also expose the tensions in decision-making regarding actions required to control and mitigate these impacts in order to provide effective protection to these ecosystems and their contained biodiversity and communities. It is clear that many different factors, both of human origin and of the natural environment, may act in synergy and disentangling their subtleties is often more challenging and complex than previously realised.
References
Agnew, D.J., and S. Nichol. 1996. Marine disturbances—commercial fishing. Foundations for ecological research west of the Antarctic Peninsula. Antarctic Research Series 70: 417–435.
Antarctic Treaty Secretariat. 2019. Management plan for Antarctic Specially Managed Area No. 7 Southwest Anvers Island and Palmer Basin. https://documents.ats.aq/recatt/att658_e.pdf Accessed 29 November, 2021
Arthur, B., M. Hindell, M. Bester, P.J.N. De Bruyn, P. Trathan, M. Goebel, and A.-M. Lea. 2017. Winter habitat predictions of a key Southern Ocean predator, the Antarctic fur seal (Arctocephalus gazella). Deep Sea Research II Topical Studies in Oceanography 140: 171–181.
Atkinson, A., S.L. Hill, E.A. Pakhomov, V. Siegel, C.S. Reiss, V.J. Loeb, D.K. Steinberg, K. Schmidt, et al. 2019. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nature Climate Change 9: 142–147.
Basberg, B.L. 2004. The Shore Whaling Stations at South Georgia. Sandefjord: Christensen’s Whaling Museum.
Bender, E.A., T.J. Case, and M.E. Gilpin. 1984. Perturbation experiments in community ecology: Theory and practice. Ecology 65: 1–13.
Bertrand, K.J. 1971. Americans in Antarctica, 1775–1948, 554. New York: American Geophysical Society.
Bester, M.N., P.G. Ryan, and B.M. Dyer. 2003. Population numbers of fur seals at Prince Edward Island, Southern Ocean. African Journal of Marine Science 25: 549–554.
Block, W., R.I.L. Smith, and A.D. Kennedy. 2009. Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biological Reviews 84: 449–484.
Bokhorst, S., P. Convey, and R. Aerts. 2019. Nitrogen inputs by marine vertebrates drive abundance and richness in Antarctic terrestrial ecosystems. Current Biology 29: 1721–1727. https://doi.org/10.1016/j.cub.2019.04.038.
Bonner, W.N. 1958. Exploitation and conservation of seals in South Georgia. Oryx 4: 373–380.
Bonner, W.N. 1968. The fur seal of South Georgia. British Antarctic Survey Scientific Reports 56. London: British Antarctic Survey.
Bonner, W.N. 1985. Impacts of fur seals on the terrestrial environment at South Georgia. In Antarctic nutrient cycles and food webs, ed. W.R. Siegfried, P.R. Condy, and R.M. Laws, 641–646. Heidelberg: Springer.
Boyd, I.L. 1993. Pup production and distribution of breeding Antarctic fur seals (Arctocephalus gazella) at South Georgia. Antarctic Science 5: 17–24.
Boyd, I.L., J.P. Croxall, N.J. Lunn, and K. Reid. 1995. Population demography of Antarctic fur seals: The costs of reproduction and implications for life-histories. Journal of Animal Ecology 64: 505–518.
Boyd, I.L., D.J. McCafferty, K. Reid, R. Taylor, and T.R. Walker. 1998. Dispersal of male and female Antarctic fur seals (Arctocephalus gazella). Canadian Journal of Fisheries and Aquatic Science 55: 845–852.
Braun, C., F. Hertel, O. Mustafa, A. Nordt, S. Pfeiffer, and H.-U. Peter. 2014. Environmental assessment and management challenges of the Fildes Peninsula Region. In Antarctic futures, ed. T. Tin, D. Liggett, P. Maher, and M. Lamers, 169–191. Dordrecht: Springer.
Braun, C., O. Mustafa, A. Nordt, S. Pfeiffer, and H.-U. Peter. 2012. Environmental monitoring and management proposals for the Fildes Region, King George Island, Antarctica. Polar Research 31: 18206.
Brooks, S.T., J. Jabour, J. van den Hoff, and D.M. Bergstrom. 2019. Our footprint on Antarctica competes with nature for rare ice-free land. Nature Sustainability 2: 185–190.
Butler, H.G. 1999. Seasonal dynamics of the planktonic microbial community in a maritime Antarctic lake undergoing eutrophication. Journal of Plankton Research 21: 2393–2419.
Butler, H.G. 2000. Temporal plankton dynamics in an oligotrophic maritime Antarctic lake. Freshwater Biology 43: 215–230.
Cannone, N., M. Dalle Fratte, P. Convey, M.R. Worland, and M. Guglielmin. 2017. Ecology of moss banks at Signy Island (maritime Antarctica). Botanical Journal of the Linnean Society 184: 518–533.
Cannone, N., M. Guglielmin, P. Convey, M.R. Worland, and S.E. Favero-Longo. 2016. Vascular plant changes in extreme environments: Effects of multiple drivers. Climatic Change 134: 651–665.
Cannone, N., F. Malfasi, S.E. Favero-Longo, P. Convey, and M. Guglielmin. 2022. Acceleration of climate warming and vascular plant expansion in maritime Antarctica. Current Biology. https://doi.org/10.1016/j.cub.2022.01.074.
Carlini, A.R., G.A. Daneri, R. Casaux, and E.I. Márquez. 2006. Haul-out patterns of itinerant male Antarctic fur seals (Arctocephalus gazella) at Laurie Island, South Orkney Islands. Polar Research 25: 139–144.
Cavanagh, R.D., J. Melbourne-Thomas, S.M. Grant, D.K.A. Barnes, K.A. Hughes, S. Halfter, M.P. Meredith, E.J. Murphy, et al. 2021. Future risk for Southern Ocean ecosystem services under climate change. Frontiers in Marine Science 7: 615214. https://doi.org/10.3389/fmars.2020.615214.
Chown, S.L., R.I. Leihy, T.R. Naish, C.M. Brooks, P. Convey, B.J. Henley, A.N. Mackintosh, L.M. Phillips, et al., eds. 2022. Antarctic climate change and the environment: A decadal synopsis and recommendations for action, 108. Cambridge: Scientific Committee on Antarctic Research.
Coetzee, B.W.T., P. Convey, and S.L. Chown. 2017. Expanding the protected area network in Antarctica is urgent and readily achievable. Conservation Letters 10: 670–680.
Constable, A.J., J. Melbourne-Thomas, S.P. Corney, K.R. Arrigo, C. Barbraud, D.K.A. Barnes, N.L. Bindoff, P.W. Boyd, et al. 2014. Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Global Change Biology 20: 3004–3025.
Convey, P. 2017. Antarctic ecosystems. Encyclopedia of Biodiversity 1: 179–187.
Convey, P. 2020. The price of cumulative human activities in the Antarctic. Antarctic Science 32: 425. https://doi.org/10.1017/S0954102020000577.
Convey, P., E.M. Biersma, A. Casanova-Katny, and C.S. Maturana. 2020. Refuges of Antarctic diversity. In Past Antarctica, ed. M. Oliva and J. Ruiz-Fernandez, 181–200. Burlington: Academic Press.
Convey, P., S.L. Chown, A. Clarke, D.K.A. Barnes, V. Cummings, H. Ducklow, F. Frati, T.G.A. Green, et al. 2014. The spatial structure of Antarctic biodiversity. Ecological Monographs 84: 203–244.
Convey, P., and M. Lebouvier. 2009. Environmental change and human impacts on terrestrial ecosystems of the sub-Antarctic islands between their discovery and the mid-Twentieth Century. Papers and Proceedings of the Royal Society of Tasmania 143: 33–44.
Convey, P., A. Morton, and J. Poncet. 1999. Survey of marine birds and mammals of the South Sandwich Islands. Polar Record 35: 107–124.
Convey, P., and L.S. Peck. 2019. Antarctic environmental change and biological responses. Science Advances 11: eaaz0888.
Cook, A.J., A.J. Fox, D.G. Vaughan, and J.G. Ferrigno. 2005. Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308: 541–544.
Croxall, J.P. 1992. Southern Ocean environmental changes: Effects on seabird, seal and whale populations. Philosophical Transactions of the Royal Society Series B 338 (319–328): 8.
Eayrs, C., X. Li, M.N. Raphael, and D.M. Holland. 2021. Rapid decline in Antarctic sea ice in recent years hints at future change. Nature Geoscience 14: 460–464. https://doi.org/10.1038/s41561-021-00768-3.
Ellis-Evans, J.C. 1990. Evidence for change in the chemistry of maritime Antarctic Heywood Lake. In Antarctic ecosystems. Ecological change and conservation, ed. K.R. Kerry and G. Hempel, 77–82. Berlin: Springer.
Favero-Longo, S.E., N. Cannone, M.R. Worland, P. Convey, R. Piervittori, and M. Guglielmin. 2011. Changes in lichen vegetation with fur seal population increase on Signy Island (South Orkney Islands, Maritime Antarctic). Antarctic Science 23: 65–77.
Finger, J.V.G., D.H. Corá, P. Convey, F.S. Cruz, and L. Krüger. 2021. Anthropogenic debris in an Antarctic Specially Protected Area in the Maritime Antarctic. Marine Pollution Bulletin 172: 112921. https://doi.org/10.1016/j.marpolbul.2021.112921.
Foley, C.M., and H.J. Lynch. 2020. A method to estimate pre-exploitation population size. Conservation Biology 34: 256–265. https://doi.org/10.1111/cobi.13416.
Forcada, J., and J.I. Hoffman. 2014. Climate change selects for heterozygosity in a declining fur seal population. Nature 511: 462–465. https://doi.org/10.1038/nature13542.
Forcada, J., and I.J. Staniland. 2009. Antarctic fur seal Arctocephalus gazella. In Encyclopedia of marine mammals, 2nd ed., ed. W.F. Perrin, B. Würsig, and J.G.M. Thewissen, 36–42. London: Academic Press.
Frenot, Y., J.C. Gloaguen, L. Massé, and M. Lebouvier. 2001. Human activities, ecosystem disturbance and plant invasions in subantarctic Crozet, Kerguelen and Amsterdam Islands. Biological Conservation 101: 33–50.
Fretwell, P.T., P. Convey, A.F. Fleming, H.J. Peat, and K.A. Hughes. 2011. Detecting and mapping vegetation distribution on the Antarctic Peninsula from remote sensing data. Polar Biology 34: 273–281.
Furse, C. 1986. Seals and whales. In Antarctic year: Brabant island expedition, 220–221. London: Croom Helm.
Goebel, M., B. McDonald, S. Freeman, R. Haner, N. Spear, and S. Sexton. 2008. Pinniped research at Cape Shirreff, Livingston Island, Antarctica. In AMLR 2007/08 Field Season Report. NOAA-TM-NMFS-SWFSC-427, ed. A. Van Cise.
Goldsworthy, S.D., J. McKenzie, B. Page, M.L. Lancaster, P.D. Shaughnessy, L.P. Wynen, S.A. Robinson, K.J. Peters, et al. 2009. Fur seals at Macquarie Island: Post-sealing colonisation, trends in abundance and hybridisation of three species. Polar Biology 32: 1473–1486.
Grant, S.M., C.L. Waller, S.A. Morley, D.K.A. Barnes, M.J. Brasier, M.C. Double, H.J. Griffiths, K.A. Hughes, et al. 2021. Local drivers of change in Southern Ocean ecosystems: Human activities and policy implications. Frontiers in Ecology and Evolution 9: 624518. https://doi.org/10.3389/fevo.2021.624518.
Griffiths, H.J., A.J.S. Meijers, and T.J. Bracegirdle. 2017. More losers than winners in a century of future Southern Ocean seafloor warming. Nature Climate Change 7: 749–754. https://doi.org/10.1038/nclimate3377.
Guinet, C., P. Jouventin, and J.-Y. Georges. 1994. Long term population changes of fur seals Arctocephalus gazella and Arctocephalus tropicalis on Subantarctic (Crozet) and subtropical (St. Paul and Amsterdam) Islands and their possible relationship to El-Niño southern oscillation. Antarctic Science 6: 473–478.
Hart, I.B. 2006. Whaling in the Falkland Islands Dependencies, 1904–1931. Pequena: Newton St. Margarets.
Haussmann, N.S., E.M. Rudolph, J.M. Kalwij, and T. McIntyre. 2013. Fur seal populations facilitate establishment of exotic vascular plants. Biological Conservation 162: 33–40.
Hawes, I. 1990. Eutrophication and vegetation development in maritime Antarctic lakes. In Antarctic ecosystems—Ecological change and conservation, ed. K.R. Kerry and G. Hempel, 83–90. Berlin: Springer.
Headland, R.K. 1984. The island of South Georgia. Cambridge: Cambridge University Press.
Headland, R.K. 1989. Chronological list of Antarctic expeditions and related historical events. Cambridge: Cambridge University Press.
Headland, R.K. 2009. A chronology of Antarctic exploration. London: Bernard Quaritch.
Headland, R.K. 2014. Evaluation and protection of Antarctic heritage sites on South Georgia. Nimrod 8: 67–85.
Headland, R.K., ed. 2018. Historical Antarctic sealing industry. Cambridge: Scott Polar Research Institute, University of Cambridge, Occasional Publication Series.
Hemmings, A.D., and L.K. Kriwoken. 2010. High level Antarctic EIA under the Madrid Protocol: State practice and the effectiveness of the Comprehensive Environmental Evaluation process. International Environmental Agreements: Politics, Law and Economics 10: 187–208.
Henderson, K.A., C.T. Bauch, and M. Anand. 2016. Alternative stable states and the sustainability of forests, grasslands, and agriculture. Proceedings of the National Academy of Sciences USA 113: 14552–14559. https://doi.org/10.1073/pnas.1604987113.
Heywood, R.B. 1967. Antarctic ecosystems: The freshwater lakes of Signy Island and their fauna. Philosophical Transactions of the Royal Society of London 261: 347–362.
Heywood, R.B., H.J.G. Dartnall, and J. Priddle. 1980. Characteristics and classification of the lakes of Signy Island, South Orkney Islands, Antarctica. Freshwater Biology 10: 47–59.
Hodgson, D.A., and N.M. Johnston. 1997. Inferring seal populations from lake sediments. Nature 387: 30–31.
Hodgson, D.A., N.M. Johnston, A.P. Caulkett, and V.J. Jones. 1998. Palaeolimnology of Antarctic fur seal Arctocephalus gazella populations and implications for Antarctic management. Biological Conservation 83: 145–154.
Hoffman, J.I., E. Bauer, A.J. Paijmans, E. Humble, L.M. Beckmann, C. Kubetschek, F. Christaller, N. Kröcker, et al. 2018. A global cline in a colour polymorphism suggests a limited contribution of gene flow towards the recovery of a heavily exploited marine mammal. Royal Society Open Science. https://doi.org/10.1098/rsos.181227.
Hofman, R.J. 2017. Sealing, whaling and krill fishing in the Southern Ocean: Past and possible future effects on catch regulations. Polar Record 53: 88–99.
Hofmeyr, G. J. G. 2016. Arctocephalus gazella. IUCN Red List of Threatened Species. 2016:eT2058A66993062.
Hofmeyr, G.J.G., M.N. Bester, A.B. Makhado, and P.A. Pistorius. 2006. Population changes in Subantarctic and Antarctic fur seals at Marion Island. South African Journal of Wildlife Research 36: 55–68.
Hofmeyr, G.J.G., B.A. Krafft, S.P. Kirkman, M.N. Bester, C. Lydersen, and K.M. Kovacs. 2005. Population changes of Antarctic fur seals at Nyrøysa, Bouvetøya. Polar Biology 28: 725–731.
Hucke-Gaete, R., L.P. Osman, C.A. Moreno, and D. Torres. 2004a. Examining natural population growth from near extinction: The case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biology 27: 304–311.
Holdgate, M.W., and P. E. Baker. 1979. The South Sandwich Islands: I. General description. British Antarctic Survey Scientific Report 91.
Hughes, K.A. 2003. Influence of seasonal environmental variables on the distribution of presumptive fecal coliforms around an Antarctic research station. Applied and Environmental Microbiology 69: 4884–4891.
Hughes, K.A., and P. Convey. 2014. Alien invasions in Antarctica—Is anyone liable? Polar Research 33: 22103.
Hughes, K.A., P. Fretwell, J. Rae, K. Holmes, and A. Fleming. 2011. Untouched Antarctica: Mapping a finite and diminishing environmental resource. Antarctic Science 23: 537–548. https://doi.org/10.1017/S095410201100037X.
Hughes, K.A., P. Convey, and J. Turner. 2021. Developing resilience to climate change impacts in Antarctica: An evaluation of Antarctic Treaty System protected area policy. Environmental Science & Policy 124: 12–22. https://doi.org/10.1016/j.envsci.2021.05.023.
Hughes, K.A., L.R. Pertierra, M.A. Molina-Montenegro, and P. Convey. 2015. Biological invasions in Antarctica: What is the current status and can we respond? Biodiversity and Conservation 24: 1031–1055.
Hughes, K.A., L. Ireland, P. Convey, and A.H. Fleming. 2016. Assessing the effectiveness of specially protected areas for conservation of Antarctica’s botanical diversity. Conservation Biology 30: 113–120.
Izaguirre, I., L. Allende, and M. Romina Schiaffino. 2021. Phytoplankton in Antarctic lakes: Biodiversity and main ecological features. Hydrobiologia 848: 177–207.
Johnstone, G. W. 1982. Zoology. Expedition to the Australian Territory of Heard Island and the MacDonald Islands, 1980. Division of National Mapping, Canberra, Australia, Technical Report 31.
Krause, D.J., C.A. Bonin, M.E. Goebel, C.S. Reiss, and G.M. Watters. 2022. The rapid population collapse of a key marine predator in the northern Antarctic Peninsula endangers genetic diversity and resilience to climate change. Frontiers in Marine Science 8: 796488. https://doi.org/10.3389/fmars.2021.796488.
Krause, D.J., and J.T. Hinke. 2021. Finally within reach: A drone census of an important, but practically inaccessible, Antarctic fur seal colony. Aquatic Mammals 47: 349–354. https://doi.org/10.1578/AM.47.4.2021.349.
Larson, C. A. 1920. Report of Interdepartmental Committees on Research and Development in the Dependencies of the Falkland Islands. Command 657, H.M.S.O., London, UK. 92 pp.
Laws, R.M. 1973. Population increase of fur seals at South Georgia. Polar Record 16: 856–858.
Laws, R.M. 1981. Seal surveys, South Orkney Islands, 1971 and 1974. British Antarctic Survey Bulletin 54: 136–139.
Laws, R.M. 1994. History and present status of southern elephant seal populations. In Elephant seals: population ecology, behaviour, and physiology, ed. B.J. Le Boeuf and R.M. Laws, 49–65. Berkley: University of California Press.
Lee, J.R., B. Raymond, T.J. Bracegirdle, I. Chadès, R.A. Fuller, J.D. Shaw, and A. Terauds. 2017. Climate change drives expansion of Antarctic ice-free habitat. Nature 547: 49–54.
Leihy, R.I., B.W.T. Coetzee, F. Morgan, B. Raymond, J.D. Shaw, A. Terauds, D. Bastmeijer, and S.L. Chown. 2020. Antarctica’s wilderness fails to capture continent’s biodiversity. Nature 583: 567–571.
Lyons, D. 2009. Environmental impact assessment in Antarctica under the Protocol on Environmental Protection. Polar Record 29: 111–120.
Malfasi, F., P. Convey, and N. Cannone. 2019. Establishment and eradication of an alien plant species in Antarctica: Poa annua at Signy Island. Biodiversity and Conservation 29: 173–186.
Marr, J.W.S. 1935. The South Orkney Islands. Discovery Report 10: 370–377.
May, R.M., J.R. Beddington, C.W. Clark, and R.M. Laws. 1979. Management of multi-species fisheries. Science 205: 267–277.
McBride, M.M., P. Dalpadado, K.F. Drinkwater, O.R. Good, A.J. Hobday, A.B. Hollowed, T. Kristiansen, E.J. Murphy, et al. 2014. Krill, climate, and contrasting future scenarios for Arctic and Antarctic fisheries. ICES Journal of Marine Science 71: 1934–1955.
McCormack, S.A., J. Melbourne-Thomas, R. Trebilco, G. Griffith, S.L. Hill, C. Hoover, N.M. Johnston, T.I. Marina, et al. 2021. Southern Ocean food web modelling: Progress, prognoses, and future priorities for research and policy makers. Frontiers in Ecology and Evolution 9: 624763. https://doi.org/10.3389/fevo.2021.624763.
Mori, A.S. 2011. Ecosystem management based on natural disturbances: Hierarchical context and non-equilibrium paradigm. Journal of Applied Ecology 48: 280–292. https://doi.org/10.1111/j.1365-2664.2010.01956.x.
Morley, S.A., D. Abele, D.K.A. Barnes, C.A. Cárdenas, C. Cotté, J. Gutt, J.S.F. Henley, J. Höfer, et al. 2020. Global drivers on Southern Ocean ecosystems: Changing physical environments in an Earth system. Frontiers in Marine Science 7: 547188. https://doi.org/10.3389/fmars.2020.547188.
Mulvaney, R., N.J. Abram, R.C.A. Hindmarsh, C. Arrowsmith, L. Fleet, J. Triest, L. Sime, et al. 2012. Recent Antarctic Peninsula warming relative to Holocene climate and ice shelf history. Nature 489: 141–144. https://doi.org/10.1038/nature11391.
Ochyra, R., R.I.L. Smith, and H. Bednarek-Ochyra. 2008. The illustrated moss flora of Antarctica. Cambridge: Cambridge University Press.
Øritsland, T. 1970. Sealing and seal research in the southwest Atlantic pack ice, September–October 1964. In Antarctic Ecology (Volume 1), ed. M.W. Holdgate, 367–374. London: Academic Press.
Øvstedal, D.O., and R.I.L. Smith. 2001. Lichens of Antarctica and South Georgia: A guide to their identification and ecology. Cambridge: Cambridge University Press.
Paijmans, A.J., M.A. Stoffel, M.A. Bester, A.C. Cleary, P.J.N. De Bruyn, J. Forcada, M.E. Goebel, S.D. Goldsworthy, et al. 2021. The genetic legacy of extreme exploitation in a polar vertebrate. Scientific Reports 10: 5089.
Payne, M.R. 1977. Growth of a fur seal population. Philosophical Transactions of the Royal Society, Series B 279: 67–79.
Pearce, D.A., C. van der Gast, K. Woodward, and K.K. Newsham. 2005. Significant changes in the bacterioplankton community structure of a maritime Antarctic freshwater lake following nutrient enrichment. Microbiology 151: 3237–3248.
Pertierra, L.R., K.A. Hughes, G.C. Vega, and M.Á. Olalla-Tárraga. 2017. High resolution spatial mapping of human footprint across Antarctica and its implications for the strategic conservation of avifauna. PLoS ONE 12: e0168280.
Peter H.-U., C. Braun, S. Janowski, A. Nordt, A. Nordt and M. Stelter. 2013. The current environmental situation and proposals for the management of the Fildes Peninsula Region. Dessau: German Federal Environment Agency. https://www.umweltbundesamt.de/sites/default/files/medien/461/publikationen/4424.pdf
Peter, H.-U., C. Buesser, O. Mustafa, and S. Pfeiffer. 2008. Risk assessment for the Fildes Peninsula and Ardley Island, and development of management plans for their designation as Specially Protected or Specially Managed Areas. Dessau: German Federal Environment Agency. https://www.umweltbundesamt.de/en/publikationen/risk-assessment-for-fildes-peninsula-ardley-island
Quayle, W.C., and P. Convey. 2006. Concentration, molecular weight distribution and carbohydrate composition of DOC in maritime Antarctic lakes of differing trophic status. Aquatic Geochemistry 12: 161–178.
Reid, K., D. Davis, and I.J. Staniland. 2006. Spatial and temporal variability in the fish diet of Antarctic fur seal (Arctocephalus gazella) in the Atlantic sector of the Southern Ocean. Canadian Journal of Zoology 84: 1025–1037. https://doi.org/10.1139/Z06-071.
Sancho, L.G., A. Pintado, F. Navarro, M. Ramos, M.A. De Pablo, J.M. Blanquer, J. Raggio, F. Valladares, et al. 2017. Recent warming and cooling in the Antarctic Peninsula region has rapid and large effects on lichen vegetation. Scientific Reports 7: 5689. https://doi.org/10.1038/s41598-017-05989-4.
SCAR. 2006. Proposal to de-list Antarctic fur seals as Specially Protected Species. Working Paper 39. Antarctic Treaty Consultative Meeting XXIX, Edinburgh, UK, 12–23 June, 2006.
SCAR. 2018. Environmental Code of Conduct for Terrestrial Scientific Field Research in Antarctica. 6pp. Retrieved 12 January, 2022 from https://www.scar.org/policy/scar-codes-of-conduct/
SCAR-EGS. 2008. Scientific Committee for Antarctic Research – Expert Group on Seals Report. http://www.seals.scar.org/pdf/statusofstocs.pdf. Accessed 17 January 2022.
Senatore, M.X. 2019. Assessing tourism patterns in the South Shetland Islands for the conservation of 19th-century archaeological sites in Antarctica. Polar Record 55: 154–168. https://doi.org/10.1017/S0032247419000391.
Shaw, J.D., A. Terauds, M.J. Riddle, H.P. Possingham, and S.L. Chown. 2014. Antarctica’s protected areas are inadequate, unrepresentative, at risk. PLoS Biology. https://doi.org/10.1371/journal.pbio.1001888.
Shears, J., and K. J. Richard. 1994. Inspection survey of Specially Protected Areas in the South Orkney Islands, Antarctica. British Antarctic Survey Report (Archive Code AD6/2H/1993/NTS).
Siegert, M., A. Atkinson, A. Banwell, M. Brandon, P. Convey, B. Davies, R. Downie, T. Edwards, et al. 2019. The Antarctic Peninsula under a 1.5 °C global warming scenario. Frontiers in Environmental Science 7: 102. https://doi.org/10.3389/fenvs.2019.00102.
Smith, R.I.L. 1972. Vegetation of the South Orkney Islands with particular reference to Signy Island. British Antarctic Survey Scientific Reports 68: 1–124
Smith, R.I.L. 1990. Signy Island as a paradigm of biological and environmental change in Antarctic terrestrial ecosystems. In: Antarctic Ecosystems, Ecological Change and Conservation Kerry K.R. and G. Hempel eds, 30–48. Springer-Verlag Berlin.
Smith, R., and S. Stammerjohn. 2001. Variations of surface air temperature and sea-ice extent in the western Antarctic Peninsula region. Annals of Glaciology 33: 493–500. https://doi.org/10.3189/172756401781818662.
Smith, R.I.L. 1988a. Destruction of Antarctic terrestrial ecosystems by a rapidly increasing fur seal population. Biological Conservation 45: 55–72.
Smith, V.R. 1988b. Production and nutrient dynamics of plant communities on a sub-Antarctic Island. Polar Biology 8: 255–269.
Smith, R.I.L. 1996a. Terrestrial and freshwater biotic components of the West Antarctic Peninsula. In Foundations of ecological research west of the Antarctic Peninsula, ed. R. Ross, E. Hofmann, and L. Quetin, 15–59. Washington, DC: American Geophysical Union.
Smith, R.I.L. 1996b. Terrestrial and freshwater biotic components of the West Antarctic. Antarctic Research Series 70: 15–59.
Smith, R.I.L. 1997. Impact of an increasing fur seal population on Antarctic plant communities: Resilience and recovery. In Antarctic communities: Species, structure and survival, ed. B. Battaglia, J. Valencia, and D.W.H. Walton, 432–436. Cambridge: Cambridge University Press.
Smith, R. I. L., D. W. H. Walton, and P. R. Dingwall. (eds.) 1994. Developing the Antarctic Protected Area System: proceedings of the SCAR/IUCN Workshop on Antarctic Protected Areas, Cambridge, UK, 29 June – 2 July 1992. Gland, Switzerland: IUCN The World Conservation Union. 137 pp.
Stammerjohn, S.E., D.G. Martinson, R.C. Smith, and R.A. Iannuzzi. 2008. Sea ice in the western Antarctic Peninsula region: Spatio-temporal variability from ecological and climate change perspectives. Deep Sea Research Part II: Topical Studies in Oceanography 55: 2041–2058.
Sun, L.G., X.D. Liu, X.B. Yin, R. Zhu, Z. Xie, and Y. Wang. 2004. A 1500-year record of Antarctic seal populations in response to climate change. Polar Biology 27: 495–501.
Tejedo, P., L. Pertierra, J. Benayas, P. Convey, A. Justel, and A. Quesada. 2012. Trampling on Maritime Antarctica Can soil ecosystems be effectively protected through existing codes of conduct? Polar Research 31: 10888.
Terauds, A., and J.R. Lee. 2016. Antarctic biogeography revisited: Updating the Antarctic Conservation Biogeographic Regions. Diversity and Distribution 22: 836–840.
Terauds, A., S.L. Chown, F. Morgan, H.J. Peat, D.J. Watts, H. Keys, P. Convey, and D.M. Bergstrom. 2012. Conservation biogeography of the Antarctic. Diversity and Distribution 18: 726–741.
Tin, T., Z. Fleming, K.A. Hughes, D. Ainley, P. Convey, C. Moreno, S. Pfeiffer, J. Scott, et al. 2009. Impacts of local human activities on the Antarctic environment: A review. Antarctic Science 21: 3–33.
Townrow, K. 1988. Sealing sites on Macquarie Island: An archaeological survey. Papers and Proceedings of the Royal Society of Tasmania 122: 15–25.
Trathan, P.N., S. Fielding, P.R. Hollyman, E.J. Murphy, V. Warwick-Evans, and M.A. Collins. 2021. Enhancing the ecosystem approach for the fishery for Antarctic krill within the complex, variable, and changing ecosystem at South Georgia. ICES Journal of Marine Science. https://doi.org/10.1093/icesjms/fsab092.
Trathan, P.N., N. Ratcliffe, and E.A. Masden. 2012. Ecological drivers of change at South Georgia: The krill surplus, or climate variability. Ecography 35: 983–993.
Trathan, P.N., and K. Reid. 2009. Exploitation of the marine ecosystem in the sub-Antarctic: Historical impacts and current consequences. Papers and Proceedings of the Royal Society of Tasmania 143: 9–14.
Turner, J., R. Bindschadler, P. Convey, G. di Prisco, E. Fahrbach, J. Gutt, D. Hodgson, P. Mayewski, et al., eds. 2009. Antarctic climate change and the environment. Cambridge: Scientific Committee on Antarctic Research.
Vergani, D.F., and N.R. Coria. 1989. Increases in numbers of male fur seals Arctocephalus gazella during the summer-autumn period at Mossman Peninsula (Laurie Island). Polar Biology 9: 487–488.
Waluda, C.M., S. Gregory, and M.J. Dunn. 2010. Long-term variability in the abundance of Antarctic fur seals Arctocephalus gazella at Signy Island, South Orkneys. Polar Biology 33: 305–312. https://doi.org/10.1007/s00300-009-0706-2.
Yang, Q.C., L.G. Sun, D.M. Kong, T. Huang, and Y. Wang. 2010. Variation of Antarctic seal population in response to human activities in the 20th century. Chinese Science Bulletin 55: 1084–1087.
Yi, C., and N. Jackson. 2021. A review of measuring ecosystem resilience to disturbance. Environmental Research Letters 16: 053008. https://doi.org/10.1088/1748-9326/abdf09/pdf.
Zerbini, A.N., G. Adams, J. Best, P.J. Clapham, J.A. Jackson, and A.E. Punt. 2019. Assessing the recovery of an Antarctic predator from historical exploitation. Royal Society Open Science 6: 190368. https://doi.org/10.1098/rsos.190368.
Zwolicki, A., M. Barcikowski, A. Barcikowski, M. Cymerski, L. Stempniewicz, and P. Convey. 2015. Seabird colony effects on soil properties and vegetation zonation patterns on King George Island, Maritime Antarctic. Polar Biology 38: 1645–1655.
Acknowledgements
P. Convey and K. Hughes are supported by NERC core funding to the BAS ‘Biodiversity, Evolution and Adaptation’ Team and Environment Office, respectively. We particularly thank M. Dunn and R.I.L Smith for helpful comments and discussion on earlier versions of the manuscript. We thank Laura Gerrish, from the Mapping and Geographic Information Centre (MAGIC), BAS, for the preparation of Figs. 3 and 4. This paper is also a contribution to the ‘Human Impacts and Sustainability’ research theme of the SCAR Scientific Research Programme ‘Integrated Science to Inform Antarctic and Southern Ocean Conservation’ (Ant-ICON). Two anonymous reviewers are thanked for their helpful comments on the manuscript.
Funding
Funding was provided by Natural Environment Research Council.
Author information
Authors and Affiliations
Contributions
The original idea for the review and first draft text was conceived by PC. Subsequently both PC and KAH jointly developed and edited the text, drawing on their respective areas of expertise in Antarctic ecosystems, conservation and environmental management.
Corresponding author
Ethics declarations
Conflict of interest
The authors identify no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Convey, P., Hughes, K.A. Untangling unexpected terrestrial conservation challenges arising from the historical human exploitation of marine mammals in the Atlantic sector of the Southern Ocean. Ambio 52, 357–375 (2023). https://doi.org/10.1007/s13280-022-01782-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-022-01782-4