Abstract
Concerns about the sustainability of inorganic fertilizers necessitate the characterization of alternative P source materials for agronomic P-efficiencies and P losses via leaching. Firstly, this study examined nutrient compositions including P speciation of seven soil amendments: sewage sludge (SS), anaerobic digestate (AD), green compost (GC), food waste compost (FWC), chicken manure (CM), biochar, and seaweed. Secondly, soil P leaching and availability was studied on a subset of four materials (SS, AD, GC, and CM). Sorption of extracts onto columns of a test soil showed strong P retention for SS and compost, but weak P sorption for CM and especially AD, suggesting short-term leaching risks for soil applied AD. Limited P desorption with water or citrate indicated sorbed P was strongly fixed, potentially limiting crop availability. These data indicate that variation in P forms and environmental behavior should be understood to maximize P usage, but minimize leaching and soil P accumulation. Hence, different alternative P source materials need differing recommendations for their agronomic management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current global phosphorus cycle is inefficient with regard to fertilizer P usage and reuse of P-containing wastes, leading to global depletion of high-quality mineral phosphate stocks (Cordell et al. 2011). A European-level budget (per capita values for the EU-15) showed that for an average 4.7 kg P year−1 net consumption, only 0.8 kg P year−1 is currently recycled (Ott and Rechberger 2012). There is a need to redistribute secondary P (i.e., non-mineral and biosphere P) back into agricultural systems to tighten P cycles (Elser 2012). Secondary P resources comprise natural organic and waste materials utilized for their fertilizer properties including human and animal excreta either combined or separated (e.g., source-separated urine, Maurer et al. 2006), agricultural, municipal and domestic wastes via composting or post-energy recovery methods such as anaerobic digestion, and soil improvers like biochar (Schnell et al. 2012).
Sewage sludge (SS) has long been applied to land as raw or processed sludges, for example, struvite precipitation (Muster et al. 2013) or thermal ashing P recovery (Verstraete et al. 2009). The EU-27 produces 10 million tons of dry solids annually, with 40 % applied to agricultural land (Roig et al. 2012). The wastewater industry is undergoing a transition toward resource recovery rather than processing to gain compliance for environmental discharges (Verstraete et al. 2009; Mitchell et al. 2012), driven both by raw material economics and tightening legislation for landfill disposal and water pollution (Sartorius et al. 2012). Realization of the embodied energy value of organic wastes is leading to increasing availability for nutrient recovery of anaerobic digestates (AD) after biogas energy production from agricultural or food waste (Mehta and Batstone 2013). Other P-containing materials have long been traditionally used, but variably studied. Manures have been the subject of numerous evaluations, but others such as seaweed considerably less so.
There is a need for improved characterization of alternative P sources in terms of their P availability and environmental interactions. Studies often address contaminant issues in soil amendments (Diaz-Cruz et al. 2009; Linderholm et al. 2012). However, Toor et al. (2006) proposed better knowledge on P solubility, partitioning and bioavailability are necessary to balance soil amendment fertilizer potential with protecting the environment from negative consequences of soil P accumulation, erosion losses, or leaching. Well-documented mineralization rates for composts and manures focus on N transformations since legislation sets agronomic loadings by N (Jin et al. 2011; Zarabi and Jalali 2013). A review on compost nutrient cycling by Prasad (2013) suggests that knowledge on P mineralization and leaching following compost amendments is limited but that application rates ought to be set on P, not N, availability.
Quantification of different P species by 31P NMR has been undertaken for SS (Frossard et al. 2002; Escudey et al. 2004; Peng et al. 2010), manure (Lehmann et al. 2005), and compost (Galvez-Sola et al. 2010) to inform on P speciation. Stutter et al. (2012) argued that current agronomic P inefficiencies have led to accumulating soil P in complexed forms that current crops cannot access. So what if the P forms in these potential alternative P sources largely remain unavailable to crops due to P speciation and/or fixation in soils? This scenario may lead to further system inefficiencies and eventual increased P transfer to waters, as postulated by Escudey et al. (2004), and requires technological developments to manage.
To date there have been limited studies using consistent methodologies across a range of alternative P supply materials to evaluate compositions, environmental interactions, and support field-scale agronomic trials data. The hypothesis of this study was that large variation in P and organic matter compositions between alternative fertilizer materials controls different environmental P behaviors and necessitates balancing beneficial aspects such as P availability against negative aspects such as P leaching. The approach to testing this aimed to (1) evaluate nutrient compositions for a wider set of seven alternative P source materials, then (2) on a subset of four materials (selected on the basis of variation in composition and likely product availability) to evaluate P leaching and sorption using column and batch studies.
Materials and methods
Methods are described here briefly with full details given in the Electronic Supplementary Materials methods section.
Sample collection
SS was collected from a city municipal processing plant (~250 000 p.e.) operating the Cambi thermo-hydrolysis process, that induces high temperatures and pressures using steam to disintegrate sludges for enhanced biogas recovery by anaerobic digestion (patent Cambi AS, Norway). The final dewatered sludge is sold as an agricultural amendment. Anaerobic digestate (AD) liquor came from an on-farm biogas plant processing a mixture of slurry and abattoir waste. Two types of compost were collected from a large commercial plant. Green compost (GC) comprises municipal and local authority green waste and is a certified product under the UK PAS100 scheme. Premium compost is the green compost amended with food waste compost (FWC). Commercial pelletized and dried chicken manure (CM) was sourced from a local garden center. Biochar comprised commercially sourced pyrolysis-treated softwood. Seaweed was washed and dried marine macroalgae of species Fucus vesiculosus and is representative of the dominant P fertilizer material in use in small-scale agriculture by Atlantic fringe communities even up to modern times.
Analytical methods
Materials were air-dried (30 °C) for analysis. Carbon and nitrogen contents were determined by combustion chromatography (Thermo Finnigan Flas EA 1112, Waltham, USA) and Total P by a NaOH fusion method (Smith and Bain 1982). Analysis of extraction solutions was done by ICP-OES for metals (Optima 5300DV, Perkin Elmer) and by automated colorimetric methods (Skalar San++, Breda, the Netherlands) for nutrients DOC, N, and P. The analytically defined fractionation of P yielded total dissolved P (TDP), dissolved reactive P (MRP; principally PO4-P), and dissolved unreactive P (MUP; principally organically complexed).
Solution Phase 31P NMR was performed on extracts (0.25 M NaOH and 0.05 M EDTA at 1:20 w/v ratio; Turner et al. 2003) of materials. Spectra were acquired using an Avance 500 II instrument (Bruker, Germany) employing a pulse delay of 2 s and a 90° pulse angle and using an internal standard of methylene diphosphonic acid (MDPA; Bedrock et al. 1994) for quantification.
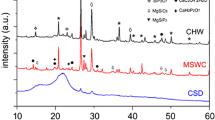
Identification of mineral phases using X-ray powder diffraction (Siemens D5000 diffractometer) was undertaken where inorganic P dominated in SS and AD samples. Identification of minerals was made on background subtracted spectra against a library of standard pure mineral phases.
Water extraction and column sorption experiments
Solution P extracts from a subset of four amendments (SS, AD, GC, and CM) were generated for use in subsequent column experiments. This selection of materials was made on the basis of (i) variation in compositional aspects of P speciation, total P, organic matter content, and (ii) current consumer uptake and future likely availability of the products for fertilizers. Water extractable P (WEP) is a common measure for assessing potential environmental P mobilization associated with soil amendments and for leaching studies (Kang et al. 2011). The extracts for the column leaching experiments were made by equilibrating 100 g each of air-dried SS, AD, CM, and GC materials with 4 L of 1 mM NaCl at room temperature on an end-over-end shaker for 16 h.
The subsets of four WEP solutions were sorbed onto duplicate glass columns (2.5 cm diameter, 6 cm length; Sigma, UK) repacked with a test soil (29 g air-dried) at 20 °C during saturated column flow experiments. The Strichen B soil (a Spodosol B horizon; described in the Electronic Supplementary Material) has previously been used as a test soil due to a strong sorption affinity for anions such as DOC and PO4 3− (Lumsdon 2004). From a background of 1 mM NaCl column, inflows were switched to the WEP solutions (1 mL min−1) and samples taken for 57–82 PVs over 22 h. Eluant samples were analyzed for P forms and UV absorbance. Sorption was studied at the pH and ionic conditions of the WEP solutions without further control. The background matrix of 1 mM NaCl approximated the ionic strength of rainwater, additionally minimizing effects due to varying ionic strength, as previously in Stutter et al. (2007, 2009).
Column material recovery and citrate extraction
Following column P loading, one column from each of the replicate pairs was destructively sampled and a back-extraction performed to examine P availability from the packing soil using (a) water and (b) citrate (Na salt, extract concentration 50 μM) in 15 mM MES buffer (2-(N-morpholino)ethanesulfonic acid) at pH 5.5 according to George et al. (2007).
Statistical analyses
Data were evaluated in Minitab (v.16). Relationships between analytical parameters were assessed using linear regression following normality testing (Andersen–Darling tests) and t tests used to determine differences between triplicate replicates of desorption with water or citrate. In Table 4, relative standard deviation is used to portray uncertainties in duplicate column leaching parameters, or triplicate back extractions from column soils. Column eluant breakthroughs were evaluated graphically. NMR spectra were processed using MestReNova (v.9.1.0).
Results
Compositions of the seven amendments are shown in Table 1 (for general properties) and Table 2 (NaOH–EDTA extracts). The largest (95 %) moisture content was for AD followed by SS, FWC, and GC. The seaweed sample was received air-dried and an assumed 80 % moisture content was used. CM and biochar were dried during the production processes. The order of total P by NaOH fusion digest (air-dried basis; Table 1) was AD > SS > CM > FWC > seaweed > GC > biochar. The greatest C content was for biochar (573 mg C kg−1) and smallest for FWC (257 mg C kg−1) and for other materials between 300 and 400 mg C kg−1. Larger N contents for sludge, AD, and CM gave low C:N ratios all of 8. SS and AD had C:P ratios <30. FWC had a smaller C:N and C:P than GC.
For NaOH–EDTA extracts, the order of TDP was sludge ≫ AD > CM > FWC > GC > seaweed ≫ biochar (Table 2). CM (58 %) and seaweed (52 %) had large contributions of organically complexed P in the extracts (MUP:TDP), with similar contributions (30–34 %) for both composts and biochar, but lower contributions for SS (12 %) and digestate (16 %). Concentrations of metals are given in Table 2. Concentrations of Fe were large in AD and GC, but small for other materials. Chicken manure, FWC, sludge, and AD had large Ca extract concentrations, with the high Ca:P ratios (>10) for biochar and FWC. Extracts of AD, seaweed, and CM had larger K than other materials.
For quantification, the 31P NMR peaks were normalized to the internal MDPA standard (Table 3; Fig. 1). Extraction efficiencies (NaOH–EDTA TDP relative to NaOH fusion; Table 1) were appropriately large for quantitative NMR (except for biochar due to low P concentrations). In terms of confidence in the quantification procedure, a highly significant relationship was attained between the NaOH–EDTA extract TDP, and the sum of peak areas of all P signals normalized to the MDPA peak area (P signal areanorm = 0.0045 × TDPNaOH–EDTA − 2.14; R 2 = 0.98, p < 0.001). Despite large organic matter contents, the dominant P form in most materials was inorganic orthophosphate P (ortho-P i ), being 88–95 % of total P, except for seaweed (72 % ortho-P i ) and CM (48 % ortho-P i ). Concentrations of organically complexed P (P o) decreased CM > SS > AD > Seaweed > FWC > GC > biochar. CM had a strong monoester P signal (Fig. 1). Monoester P dominated P o generally, except AD contained an appreciable amount of diester P and SS contained pyrophosphate P and some phosphonate. There was a significant relationship between the % P o compounds to total P determined by NMR and the % MRP of TDP determined by colorimetry on extract solutions (P o by NMR = 0.89 × %MRP − 11.2; R 2 = 0.79, p < 0.01).
Despite large concentrations of ortho-P i determined by 31P NMR in both SS and AD, the XRD analysis showed no identifiable crystalline mineral phosphate forms (Electronic Supplementary Material, Figs. S1, S2). Both samples contained quartz and calcite, with NaCl and KCl salts in the AD sample (corresponding to high Ca in both materials and K for AD in extracts; Table 3). Additionally, barite may indicate oil industry waste in SS.
Four air-dried materials were extracted into a 1 mM NaCl solution to determine water extractable P (WEP; Table 4a). The P solubility (% of total P) ranged from CM (18 %) > AD (5 %) > GC (3 %) > SS (0.3 %). In AD and CM, the WEP was dominantly molybdate unreactive (MUP; mainly organic P), with an indication of concentrated soluble dissolved organic matter (especially CM, shown by UV absorbance at 285 nm). Conversely, for GC and SS extracts, lower WEP concentrations comprised dominantly molybdate reactive P (MRP; mainly inorganic) with lower UV absorbance. Potentially leachable trace metals are shown in Table S1 (Electronic Supplementary Material). Water extract concentrations were low for Cd, Cr, and Ni but appreciable for Cu and Zn in CM.
Column breakthrough curves (BTCs) are shown in Fig. S3 and column parameters are shown in Table 4b. Leaching the WEP solutions onto columns of strongly P sorbing test soil for 57–82 column pore volumes (PV) gave final C/C 0 values of 0.1 for SS, 0.4 for GC, with column P breakthrough (i.e., C/C 0 ~ 1) only attained with feed solutions of AD and CM. Duplicate columns showed good general agreement in leaching behavior. Sewage WEP had the lowest MRP concentration (1 mg L−1) and attained the lowest C/C 0 of 0.1. The BTC strongly plateaued after 10 PVs and the soil retained 90 % of the MRP loading. Digestate extract had 4 mg MRP L−1, attained C/C 0 of 1.1 with a linear BTC and retention of 4 % of MRP loading. Green compost had extract 2 mg MRP L−1, attained C/C 0 of 0.4 with a slightly curved BTC and retention of 69 % of MRP loading. CM WEP had 7.3 mg MRP L−1, high UV absorbance and attained MRP C/C 0 of 1 with a linear BTC and 28 % retention of MRP loading. Using UV absorbance as a surrogate of DOC maximum, all columns attained maximum eluent concentrations for UV within 20 PVs at C/C 0 of 0.87 for SS, 0.91 for AD, 0.96 for GC, and 0.98 for CM. Initial extract pH ranged from 6.4 (SS) to 6.9 (GC), although pH was not determined in eluents due to small sample volumes, and UV is sensitive to pH change.
Subsequent P desorption in batch systems (as a % of P-loaded onto the column packing soils) was only appreciable for AD where 5 and 6 % of P was desorbed using water and 50 mM citrate, respectively. This was despite the AD WEP loading (as a product of low overall % retention) giving the lowest overall mass of P sorbed onto the test soil. In all other cases, P desorption was ≪1 % of extract P sorbed. Low P concentrations gave large errors in replicates (n = 3), and no differences between desorption with citrate relative to water were significant at the p < 0.05 level using t tests.
Discussion
Organic amendment P compositions and availability
The greatest total P content in the present study was for SS (21 g P kg−1 dry mass), comparable to 27 ± 9 (to a maximum 55) g P kg−1 for sludges from 48 Swedish wastewater works (Eriksson 2001). Sludge compositions (Table 1) were similar in terms of dry matter, OM, C/N, total N, and P to those (also processed by anaerobic digestion) documented by Roig et al. (2012). Peng et al. (2010) reported solution 31P NMR data for sludges from 13 Chinese urban wastewater works and suggested that spectroscopic P-speciation studies complimented enzyme P studies in looking at potential bioavailability of P o and P i . These authors showed sludge P species compositions comprised primarily inorganic ortho-phosphates with appreciable orthophosphate monoesters, similar to the current study (Table 3). Additional these authors observed minor contributions of the more labile P o forms pyrophosphates and diesters in several samples. The current evidence of a dominance of P i by solution NMR and MRP labile solution P in water and NaOH–EDTA extracts suggests high ‘potentially available’ P in SS that could be realized according to the extent of interactions with receiving soils.
Characterization is needed to inform appropriate utilization of the growing quantities of the by-product of biogas generation (anaerobic digestate; AD). Möller and Müller (2012) in a comprehensive review of AD in Germany reported large concentration ranges for dry matter (DM, 1.5–13 %), total N (3–14 % of DM), total C (36–45 % of DM), and total P (0.6–1.7 % of DM), reflecting a wide range in AD feedstocks. The AD in the present study from farm slurry and abattoir feedstock (Table 1) had nutrient contents at the low end of these ranges, but a large C:N ratio. The AD P species were dominated by inorganic orthophosphate P with minor contributions of monoesters and diesters (6 and 2 % of total P). The extracted NaOH–EDTA P was dominated (84 %) by MRP, but water solubilised P was dominated (80 %) by dissolved molybdate unreactive P (MUP), possibly influenced by soluble diester P. Möller and Müller (2012) summarized the transformations of nutrients during the AD process as a reduction in DM, OM, and C:N ratio, and found no change in total N but an increasing dominance of ammonium. For P, a loss in total P was attributed to increased pH, mineralization, and precipitation reactions involving Ca- and Mg- phosphates (e.g., struvite).Mehta and Batstone (2013) reported <10 % of total P in AD to be soluble, yet completely extracted in citrate, and they concluded P released by digestion was bound to inorganic compounds or sorbed onto solid surfaces. Although precipitation reactions limit P i solubility, no crystalline P minerals were observed in the current dried sample (Fig. S2). In this study, AD liquor was air-dried and then re-extracted into water. However, bulky organic amendments can be considered not economically viable to transport in their raw state. Drying of AD now occurs in 1 % of German biogas plants (Möller and Müller 2012).
Total P contents of the composts agree with those reported by Frossard et al. (2002) for 15 alkaline composts (urban, garden, and farm manure substrates) of mean 3.4 (range 2.1–7.2) g P kg−1 dry matter. In the present study, FWC has greater P (and N) content but lower C than GC (Table 1). Resulting low C:P and C:N suggest greater N and P mineralization and potential mobilization when FWC is added to soils. Phosphorus species were similar between the composts with slightly greater monoester P o forms in FWC (11 %) than GC (8 %; Table 3). Although NaOH–EDTA P compositions had an appreciable MUP content (30 and 33 % for GC and FWC, respectively), the water soluble P (Table 4; determined for GC only) was exclusively MRP. Frossard et al. (2002) concluded that 30–50 % of P i in composts was rapidly plant available, with another 30–50 % of P i formed of Ca-precipitates, although they urged caution that air-drying of materials could have increased P mineralization and hence P i quantification.
CM had the third largest P (and N) content (after SS and AD; Table 1), but the greatest contribution of monoester P o (Table 3) and a corresponding large fraction of MUP in NaOH–EDTA (Table 2) and water extracts (Table 4a). Many studies have used 31P NMR analysis to examine the P forms in manures (Peirce et al. 2013 and references therein). Often the specific form of inositol hexaphosphate dominates monoesters as it is not broken down in the gut of non-ruminants. Peirce et al. (2013) found that monoester P contributed about half of the P total in fresh manures, similar to the current study. These authors studied how monoester to inorganic orthophosphate ratios of chicken manures subsequently declined following the on-farm practice of manure stockpiling over increasing times, similar to observed mineralization of monoesters in soils.
There has been considerable interest in biochar as a soil conditioner and nutrient source. Although compositions vary greatly, it has been reported that 10–60 % of P is leachable from wood-derived biochar (Muckherjee and Zimmerman 2013). However, the pine-derived commercial biochar used in the present study had very small N and P contents (Table 1), with P speciation not resolvable by NMR. Large concentrations of Ca and Fe in the NaOH–EDTA extracts suggest potential for a high degree of interaction of P with bridging cations with the implication of P being more strongly bound. On the other hand, high C contents may lead to high DOC solubility and competitive sorption to maintain solubility of any small amount of P leached.
Natural materials such as seaweed have been used historically as soil amendments but lack current scientific evaluation of their P contents and speciation. Haslam and Hopkins (1996) focused on soil conditioning properties, reporting that kelp-amended soils had greater aggregate stability and enhanced microbial respiration and N mineralization rates at moderate applications. The current study showed monoesters contributed a quarter of total P with overall P contents similar to composts. Seaweed has potential as a P alternative given the abundant potential for supply globally, either directly or following use as an AD feedstock. Table 2 shows high K and Mg content which will add benefits when applied to the soil. However, investigations should be made of the consequences of associated marine-derived metal contaminant loading to soils (such as As).
A short pulse delay time (2 s) was used in NMR analysis. This parameter is important for attaining quantitative spectra, for example, McDowell et al. (2006) showed for soils that short delay times bias the results in favor of nuclei such as orthophosphate that relaxes quickly, and hence apparent diesters’ concentrations decrease. The time of relaxation is enhanced in the presence of Fe and Mn. Ratios of P/(Fe + Mn) in Table 2 show that under-representation of diesters may have been an issue for SS and CM.
Potential leaching behavior of P from use of amendments
Solubility is a factor in crop availability of P species, but P leaching represents a concern on application or accumulation of P-rich organic materials in soils. In agronomic applications, such alternative fertilizer materials would be applied to soils at standard P loading rates. Hence, an improved future approach to leaching behavior may utilize fixed P mass: extract volumes (as opposed to the fixed total mass: volume approach used here) as preparation for soil leaching experiments. However, the WEP concentration order differed greatly from that of source material P contents. The WEP for CM and AD showed high P solubility but dominated by MUP (Table 4), which may be related to the organic P forms evident by NMR of monoester P for CM and diester P for AD, with the latter generally highly soluble (Table 3). Conversely, SS and GC (with NMR showing P dominated by inorganic orthophosphate; Table 3) had relatively insoluble P, dominated by MRP and exhibited strong sorption of these dilute P extracts onto the test soil (Table 4). Sorption interactions between phosphate anions and Fe, Al surface complexes (that dominate sorption in such acid Spodosols) are affected by solution pH and sorption competition with dissolved organic matter. The pH of the water extracts occupied a narrow range (pH 6.4–6.9), being relatively neutral compared with the soil pH of 4.7 (in CaCl2). So pH differences between amendment extracts would not explain sorption behavior. It was not possible to measure the DOC concentration due to analytical issues with excessive dilutions but UV absorbance (UVabs) was large for CM indicative of soluble aromatic humic substances. Competitive sorption between organic matter and P anions (Kang et al. 2011) would be expected to explain limited sorption of 28 % of extracted manure P onto the soil. However, the most limited (4 %) sorption of extracted AD P occurred at much smaller UVabs and suggested that the P speciation limited interaction of AD WEP with Fe and Al surfaces in such soils.
Precipitation reactions may also impact on P solubility. Frossard et al. (2002) found the presence of sparingly soluble, but poorly crystallized Ca–P forms (apatites, octocalcium phosphates) in composts, which they proposed were related to Ca content but also to the presence of organic substances inhibiting crystallization. The solubility of inorganic P forms is governed by associations with organic matter and other cations forming complexes or mineral phases. The molar Ca:P ratios (derived from Table 2) were close to unity but no crystalline phosphate minerals were observed by XRD in SS or AD, only calcite. Previously Frossard et al. (2002) observed a complex mixture of octocalcium phosphates, apatites, with additionally monetite in anaerobically digested sludge.
Column systems were chosen rather than batch systems to simulate field P leaching risk since columns provide a representation of breakthrough gained from multiple samples in time and integrating physico-chemical aspects of leaching behavior (Stutter et al. 2007). Water extracts had different P concentrations and using columns experienced different loadings of P. The breakthrough curves (Fig. S3) show C/C 0 = 1 attained for AD and CM. Extrapolating these simulations to field conditions would approximate to P breakthrough for AD and CM in ~2000 mm rain (1–2 years amount) for 6 cm soil depth. Attainment of equilibria for input and output concentrations suggests saturation of the surfaces but this occurred at very different total P loadings of 11 and 82 mg P kg−1 soil for AD and CM extracts, respectively (Table 4). At the end of the column loading, C/C 0 concentrations of 0.1 and 0.4 had been attained for SS and GC, respectively. Hence, P sorption for these extracts was capable of continuing beyond the final loadings of 37 and 54 mg P kg−1 soil. Due to the complex set-up and large number of samples, generated column experiments are seldom replicated, yet the results here showed close agreement between the column duplicates in terms of the breakthrough plots (Fig. S3), C/C 0, and total P mass loadings (<10 % relative standard deviations; Table 4b).
The batch P desorption of soil recovered from the P-loaded columns evaluated reversibility of P fixation. One column of each pair was destructively sampled, once similarities in loadings and breakthrough of the duplicate columns were confirmed. The only appreciable desorption with water occurred for AD (5 % of sorbed P re-released), with limited additional release using 50 µM citrate (Table 4). Taken together these column sorption and desorption, experiments suggest that SS and GC would likely leach relatively small concentrations of MRP that is strongly and irreversibly sorbed by strongly reactive soils (such as Spodosols with large reactive Fe, Al concentrations). CM represents a greater leaching risk for soluble P (principally MUP) and has weaker interactions with soils due in part to high DOC concentrations. AD gave the greatest P leaching risk with a moderately high solubility and very limited soil sorption. Hence, these four alternative P amendments have very different P leaching risks and especially for AD would need strategies in place to minimize leaching, such as limiting applications to periods of crop growth. There are caveats to these findings such as the pre-treatment of drying and resolubilizing materials such as the AD. Such drying and rewetting may be expected to increase pools of soluble P, for example, associated with lysed microbial cells (Blackwell et al. 2009). However, drying pre-treatments may be part of necessary handling for bulky organic amendments.
Negative consequences of leaching have been long recognized for manures. Kang et al. (2011) examined P leaching from columns of sandy soils under various manure fertilizer and inorganic P additions. Recovery of MRP relative to added water extractable P was 60 % for inorganic P, but >100 % for manures, and these authors explained this in terms of P o mineralization and competitive sorption of DOC. There is a need to extend leaching behavior studies to new waste streams such as AD. Möller and Müller (2012) argued that although German biogas plants produce 66 million m3 of digestate (74 Gg P), ‘no studies about the effects of AD on P losses via leaching and runoff after field application could be found.’ Walsh et al. (2012) examined additions of chemical fertilizer, undigested slurry, and AD liquor (from a slurry feedstock similar to the material in the present study) at matched N additions onto grassland and measured soil solutions for 10 weeks post application. They found that AD and slurry gave significantly lower NO3 and NH4 leaching, but did not measure P leaching.
Conclusions
Alternative P sources in agriculture need to be characterized for P leaching and availability to maintain agronomic productivity but control environmental P losses to waters. The seven materials tested had two orders of magnitude difference in dry mass P content and differed in P speciation, C:N and C:P stoichiometry, trace and major metal contents, and physical handling properties in raw states. These variations in nutrient contents, forms, and stoichiometry have consequences for utilization of materials to realize the P content for crop growth in terms of biogeochemical cycling in soils through microbial decomposition, chemical solubility, and root uptake pathways. A subset of four air-dried materials selected on likelihood of fertilizer substitution revealed varying P solubility in artificial rainwater in terms of molybdate reactive (principally inorganic P) and unreactive solution P (principally organic P), the latter corresponding with source material organic P forms observed by 31P NMR. Interactions with an iron-rich test soil during column flow simulations of field leaching showed SS and compost were strongly sorbed, yet CM and especially AD were weakly sorbed and exhibited P breakthroughs. Batch P desorption to water or citrate was limited but greatest for AD (<5 % of sorbed P). Hence, utilizing alternative ‘waste’ materials with different compositions as replacement P fertilizers will necessitate different management strategies to minimize P leaching risk and ensure availability for crop uptake by limiting competitive soil P fixation.
References
Bedrock, C.N., M.V. Cheshire, J.A. Chudek, B.A. Goodman, and C.A. Shand. 1994. P-31 NMR studies of humic acid from a blanket peat. In Humic substances in the global environment and implications on human health, ed. N. Senesi, and T.M. Miano, 227–232. Dordrecht: Elsevier.
Blackwell, M.S.A., P.C. Brookes, N. de la Fuente-Martinez, P.J. Murray, K.E. Snars, J.K. Williams, and P.M. Haygarth. 2009. Effects of soil drying and rate of re-wetting on concentrations and forms of phosphorus in leachate. Biology and Fertility of Soils 45: 635–643.
Cordell, D., A. Rosemarin, J.J. Schröder, and A.L. Smit. 2011. Towards global phosphorus security: A system framework for phosphorus recovery and reuse options. Chemosphere 84: 747–758.
Díaz-Cruz, M.S., M.J. García-Galán, P. Guerra, A. Jelic, C. Postigo, E. Eljarrat, M. Farré, M.J. López de Alda, et al. 2009. Analysis of selected emerging contaminants in sewage sludge. Trends in Analytical Chemistry 28: 1263–1275.
Elser, J.J. 2012. Phosphorus: A limiting nutrient for humanity? Current Opinion in Biotechnology 23: 833–838.
Eriksson, J. 2001. Concentrations of 61 trace elements in sewage sludge, farmyard manure, mineral fertiliser, precipitation and in soil and crops. No. 5159. Stockholm: Swedish Environmental Protection Agency.
Escudey, M., G. Galindo, K. Avendano, D. Borchardt, A.C. Chang, and M. Briceno. 2004. Distribution of phosphorus forms in Chilean soils and sewage sludge by chemical fractionation and P-31-NMR. Journal of the Chilean Chemical Society 49: 219–222.
Frossard, E., P. Skrabal, S. Sinaj, F. Bangerter, and O. Traore. 2002. Forms and exchangeability of inorganic phosphate in composted solid organic wastes. Nutrient Cycling in Agroecosystems 62: 103–113.
Galvez-Sola, L., J. Morales, A.M. Mayoral, F.C. Marhuenda-Egea, E. Martinez-Sabater, M.D. Perez-Murcia, M.A. Bustamante, C. Paredes, et al. 2010. Estimation of phosphorus content and dynamics during composting: Use of near infrared spectroscopy. Chemosphere 78: 13–21.
George, T.S., R.J. Simpson, P.J. Gregory, and A.E. Richardson. 2007. Differential interaction of Aspergillus niger and Peniophora lycii phytases with soil particles affects the hydrolysis of inositol phosphates. Soil Biology & Biochemistry 39: 793–803.
Haslam, S.F.I., and D.W. Hopkins. 1996. Physical and biological effects of kelp (seaweed) added to soil. Applied Soil Ecology 3: 257–261.
Jin, V.L., M.V.V. Johnson, R.L. Haney, and J.G. Arnold. 2011. Potential carbon and nitrogen mineralization in soils from a perennial forage production system amended with class B biosolids. Agriculture Ecosystems and the Environment 141: 461–465.
Kang, J., A. Amoozegar, D. Hesterberg, and D.L. Osmond. 2011. Phosphorus leaching in a sandy soil as affected by organic and inorganic fertilizer sources. Geoderma 161: 194–201.
Lehmann, J., Z.D. Lan, C. Hyland, S. Sato, D. Solomon, and Q.M. Ketterings. 2005. Long term dynamics of phosphorus forms and retention in manure amended soils. Environmental Science and Technology 39: 6672–6680.
Linderholm, K., A.-M. Tillman, and J.E. Mattsson. 2012. Life cycle assessment of phosphorus alternatives for Swedish agriculture. Resources, Conservation and Recycling 66: 27–39.
Lumsdon, D.G. 2004. Partitioning of organic carbon, aluminium and cadmium between solid and solution in soils: Application of a mineral–humic particle additivity model. European Journal of Soil Science 55: 271–285.
Maurer, M., W. Pronk, and T.A. Larsen. 2006. Treatment process for source separated urine. Water Research 40: 3151–3166.
McDowell, R.W., I. Stewart, and B.J. Cade-Menun. 2006. An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy. Journal of Environmental Quality 35: 293–302.
Mehta, C.M., and D.J. Batstone. 2013. Nutrient solubilization and its availability following anaerobic digestion. Water Science and Technology 67: 756–763.
Mitchell, C., D. Fam, and D. Cordell. 2012. Effectively managing the transition towards restorative futures in the sewage industry: A phosphorus case study. In Water sensitive cities, ed. C. Howe, and C. Mitchell, 83–96. London: IWA Publishing.
Möller, K., and T. Müller. 2012. Effects of anaerobic digestate nutrient availability and crop growth: A review. Engineering in Life Sciences 12: 242–257.
Muckherjee, A., and A.R. Zimmerman. 2013. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 193–194: 122–130.
Muster, T.H., G.B. Douglas, N. Sherman, A. Seeber, N. Wright, and N. Güzükara. 2013. Towards effective phosphorus recycling from wastewater: Quantity and quality. Chemosphere 91: 676–684.
Ott, C., and H. Rechberger. 2012. The European phosphorus balance. Resource Conservation and Recycling 60: 159–172.
Peirce, C.A.E., R.J. Smernik, and T.M. McBeath. 2013. Phosphorus availability in chicken manure is lower with increasing stockpiling period, despite a larger orthophosphate content. Plant and Soil 373: 359–372.
Peng, X.L., L. Chen, Z.W. Lv, G.J. Hao, and H.L. Fang. 2010. Characterisation of phosphorus in sewage sludge from different sources by 31P Nuclear Magnetic Resonance spectroscopy. Communications in Soil Science and Plant Analysis 41: 1237–1244.
Prasad, M. 2013. A literature review on the availability of phosphorus from compost in relation to the nitrate regulations SI378 of 2006. Small scale study report prepared for the Environmental Protection Agency by Cre-composting Association of Ireland, STRIVE-program, Republic of Ireland.
Roig, N., J. Sierra, E. Marti, M. Nadel, M. Schuhmacher, and J.L. Doming. 2012. Long-term amendment of Spanish soils with sewage sludge: Effects on soil functioning. Agriculture, Ecosystems & Environment 158: 41–48.
Sartorius, C., J. von Horn, and F. Tettenborn. 2012. Phosphorus recovery from wastewater—Expert survey on present use and future potential. Water Environment Research 84: 313–322.
Schnell, R.W., D.M. Vietor, T.L. Provin, C.L. Munster, and S. Capareda. 2012. Capacity of biochar application to maintain energy crop productivity: Soil chemistry, Sorghum growth and runoff water quality effects. Journal of Environmental Quality 41: 1044–1051.
Smith, B.F.L., and D.C. Bain. 1982. A sodium hydroxide fusion method for the determination of total phosphate in soils. Communications in Soil Science and Plant Analysis 13: 185–190.
Stutter, M.I., D.G. Lumsdon, and V. Thoss. 2007. Physico-chemical and biological controls on dissolved organic matter in peat aggregate columns. European Journal of Soil Science 58: 646–657.
Stutter, M.I., S.J. Langan, and D.G. Lumsdon. 2009. Vegetated buffer strips can lead to increased release of phosphorus to water: A biogeochemical assessment of the mechanisms. Environmental Science and Technology 43: 1858–1863.
Stutter, M.I., C.A. Shand, T.S. George, M.S.A. Blackwell, R. Bol, R.L. MacKay, A.E. Richardson, L.M. Condron, et al. 2012. Recovering phosphorus from soil—A root solution?. Environmental Science and Technology 46: 1977–1978.
Toor, G.S., S. Hunger, J.D. Peak, J.T. Sims, and D.L. Sparks. 2006. Advances in the characterisation of phosphorus in organic wastes: Environment and agronomic applications. Advances in Agronomy 89: 1–72.
Turner, B.L., N. Mahieu, and L.M. Condron. 2003. The phosphorus composition of temperate pasture soils determined by NaOH-EDTA extraction and solution P-31 NMR spectroscopy. Organic Geochemistry 34: 1199–1210.
Verstraete, W., P. Caveye, and V. Diamantis. 2009. Maximum use of resources in domestic ‘used’ water. Bioresource technology 100: 5537–5545.
Walsh, J.J., D.L. Jones, G. Edwards-Jones, and A.P. Williams. 2012. Replacing inorganic fertiliser with anaerobic digestate may maintain agricultural productivity at less environmental cost. Journal of Plant Nutrition and Soil Science 175: 840–845.
Zarabi, M., and M. Jalali. 2013. Nitrogen mineralization in two calcareous soils treated with raw organic amendments. Clean Technology and Environmental Policy 15: 317–331.
Acknowledgments
The author acknowledges the analysis of samples for 31P NMR by Dr. R.L. Mackay at Dundee University and Dr. S. Hillier at James Hutton Institute for analysis and interpretation by XRD. This work was funded by the Scottish Government’s Rural Environment Research and Analysis Services. The author is grateful to the three commercial organizations which supplied the compost materials, anaerobic digestate, and sewage sludge.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Stutter, M.I. The composition, leaching, and sorption behavior of some alternative sources of phosphorus for soils. AMBIO 44 (Suppl 2), 207–216 (2015). https://doi.org/10.1007/s13280-014-0615-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-014-0615-7