Abstract
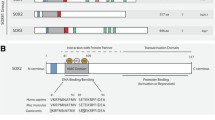
The gene encoding Sal-like 4 (Drosophila) (SALL4) is a zinc-finger transcriptional factor and a vertebrate orthologous of the Drosophila gene spalt (sal), which is upregulated in some cancers. SALL4 is expressed in the early developmental stages of Drosophila. Moreover, murine SALL4 plays a vital role in protecting the properties of embryonic stem (ES) cells and guiding the outcome of the primal inner cell mass by interacting with OCT4 and NANOG. SALL4 in ES cells and tumor cells is known as a regulator for controlling cell growth, proliferation, and apoptosis. However, the downstream goals of SALL4 remain largely uncharted. SALL4 expression has been detected in various cancers, including a subset (30 %) of solid tumors, such as breast cancer (BCa), ovarian cancer, gastric cancer, Wilms tumor, and germ cell tumors. A study has reported that SALL4 expression is commonly upregulated in human breast tumors (~86 %) and that overregulation of this gene is often linked to tumor progression. In this review, we provide an overview concerning the role of SALL4 in BCa development and progression. Furthermore, this review may identify some drugs/inhibitors for the development of BCa-specific therapies by targeting SALL4. In the future, SALL4 may be a new biomarker as a diagnostic/therapeutic target of BCa, which would be a new direction in targeted BCa therapy. To our knowledge, this is the first review of the role of SALL4 in BCa development and progression.


Similar content being viewed by others
References
Kohlhase J, Schuh R, Dowe G, et al. Isolation, characterization and organ-specific expression of two novel human zinc finger genes related to the Drosophila gene Spalt. Genomics. 1996;38:291–8.
Kohlhase J, Hausmann S, Stojmenovic G, et al. SALL3, a new member of the human Spalt-like gene family, maps to 18q23. Genomics. 1999;62:216–22.
Al-Baradie R, Yamada K, St Hilaire C, et al. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am J Hum Genet. 2002;71:1195–9.
Kuhnlein RP, Schuh R. Dual function of the region- specific homeotic gene Spalt during drosophila tracheal system development. Development. 1996;122:2215–23.
Warren M, Wang W, Spiden S, et al. A SALL4 mutant mouse model useful for studying the role of SALL4 in early embryonic development and organogenesis. Genesis. 2007;45:51–8.
Sweetman D, Munsterberg A. The vertebrate Spalt genes in development and disease. Dev Biol. 2006;293:285–93.
Ma Y, Cui W, Yang J, et al. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108(8):2726–35.
Kohlhase J. SALL4-related disorders. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews™ [internet]. Seattle (WA): University of Washington, Seattle; 2004. p. 1993–2013 [updated 2008 Mar 12].
Kohlhase J, Schubert L, Liebers M, et al. Mutations at the SALL4 locus on chromosome 20 result in a range of clinically overlapping phenotypes, including Okihiro syndrome, Holt-Oram syndrome, acro-renal-ocular syndrome, and patients previously reported to represent thalidomide embryopathy. J Med Genet. 2003;40:473–8.
Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortes J, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106(8).
Kobayashi D, Kuribayashi K, Tanaka M, Watanabe N. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncol Rep. 2011;26(4):965–70.
Cao D, Guo S, Allan RW, Molberg KH, Peng Y. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am J Surg Pathol. 2009;33(6):894–904.
Ushiku T, Shinozaki A, Shibahara J, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34(4):533–40.
Deisch J, Raisanen J, Rakheja D. Immunoexpression of SALL4 in Wilms tumors and developing kidney. Pathol Oncol Res. 2011;17(3):639–44.
Cao D, Liu A, Wang F, Allan RW, Mei K, Peng Y, Du J, Guo S, Abel TW, Lane Z, Ma J, Rodriguez M, Akhi S, Dehiya N, Li J. RNA-binding protein LIN28 is a marker for primary extragonadal germ cell tumors: an immunohistochemical study of 131 cases. Mod Pathol. 2011;24(2):288–96.
Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol. 2010;34(5):697–706.
Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009;33(7):1065–77.
Kohlhase J, Heinrich M, Schubert L, Liebers M, Kispert A, Laccone F, Turnpenny P, Winter RM, Reardon W. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002;11(23):2979–87.
Sakaki-Yumoto M et al. The murine homolog of SALL4, a causative gene in okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development. 2006;133:3005–13.
Sweetman D, Smith T, Farrell ER, Chantry A, Munsterberg A. The conserved glutamine-rich region of chick csal1 and csal3 mediates protein interactions with other Spalt family members. Implications for Townes-Brocks syndrome. J Biol Chem. 2003;278:6560–6.
Rao S, Zhen S, Roumiantsev S, McDonald LT, Yuan GC, Orkin SH. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol Cell Biol. 2010;30(22):5364–80.
Yupo Ma, Wei Cui, Jianchang Yang, Jun Qu, Chunhui Di, Hesham MA, Raymond L, Jerome R, Diane SK, Li C. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108(8): 2726–2735.
Böhm J, Sustmann C, Wilhelm C, Kohlhase J. SALL4 is directly activated by TCF/LEF inthe canonical Wnt signaling pathway Biochem Biophys Res Commun. 2006; 348(3):898–907.
Kohlhase J, Chitayat D, Kotzot D, Ceylaner S, Froster U, Fuchs S, Montgomery T, Rösler B. SALL4 mutations in Okihiro syndrome (Duane-radial ray syndrome), acro-renal-ocular syndrome, and related disorders. Hum Mutat. 2005;26:176–183.
Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:16438–16443.
Zhang J, Tam W-L, Tong GQ, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123.
Zhang L, Yan Y, Jiang Y, Cui Y, Zou Y, Qian J, Luo C, Lu Y, Wu X. The expression of SALL4 in patients with gliomas: high level of SALL4 expression is correlated with poor outcome. J Neurooncol. 2015; 121(2):261–8.
Yong KJ, Gao C, Lim JS, Yan B, Yang H, Dimitrov T, Kawasaki A, Ong CW, Wong KF, Lee S, Ravikumar S, Srivastava S, Tian X, Poon RT, Fan ST, Luk JM, Dan YY, Salto-Tellez M, Chai L, Tenen DG. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013; 368(24):2266–76.
Liu L, Zhang J, Yang X, Fang C, Xu H, Xi X. SALL4 as an Epithelial Mesenchymal Transition and Drug Resistance Inducer through the Regulation of c-Myc in Endometrial Cancer. PLoS One. 2015;10(9):e0138515.
Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33(48):5491–500.
Itou J, Matsumoto Y, Yoshikawa K, Toi M. Sal-like 4 (SALL4) suppresses CDH1 expression and maintains cell dispersion in basal-like breast cancer. FEBS Lett. 2013;587(18):3115–21.
Takashima S, Kadowaki M, Aoyama K, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011; 208:285–294.
Kobayashi D, Kuribayshi K, Tanaka M, Watanabe N. SALL4 is essential for cancer cell proliferation and is overexpressed at early clinical stages in breast cancer. Int J Oncol. 2011;38(4):933–9.
Yue X, Xiao L, Yang Y, Liu W, Zhang K, Shi G, Zhou H, Geng J, Ning X, Wu J, Zhang Q. High cytoplasmic expression of SALL4 predicts a malignant phenotype and poor prognosis of breast invasive ductal carcinoma. Neoplasma. 2015;62(6):980–8.
Jha BP Chintamani, Bhandari V, Bansal A, Saxena S, Bhatnagar D. The expression of mismatched repair genes and their correlation with clinicopathological parameters and response to neo-adjuvant chemotherapy in breast cancer Int. Semin. Surg. Oncol. 2007;4:5.
Bard JD, Gelebart P, Amin HM, Young LC, Ma Y and Lai R. Signal transducer and activator of transcription 3 is a transcriptional factor regulating the gene expression of SALL4. FASEB J. 2009; 23:1405–1414.
Dimri GP, Martinez JL and Jacobs JJ, et al. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62: 4736–4745.
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang C, Wu S, Yu D, Huang Z, Liu F, Luo Q, Yang CJ, Ouyang G. MicroRNA-33b Inhibits Breast Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci Rep. 2015; 5:9995.
Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013, 57: 1469–83.
Gao C, Kong NR, Li A, Tatetu H, Ueno S, Yang Y, He J, Yang J, Ma Y, Kao GS, Tenen DG, Chai L. SALL4 is a key transcription regulator in normal human hematopoiesis. Transfusion. 2013;53(5):1037–49.
Yang J, Chai L, Gao C, Fowles TC, Alipio Z, Dang H, Xu D, Fink LM, Ward DC, Ma Y. SALL4 is a key regulator of survival and apoptosis in human leukemic cells. Blood. 2008 ;112(3):805–13.
Liu L, Liu L, Leung LH, Cooney AJ, Chen C, Rosengart TK, Ma Y, Yang J. Knockdown of SALL4 Protein Enhances All-trans Retinoic Acid-induced Cellular Differentiation in Acute Myeloid Leukemia Cells. J Biol Chem. 2015 Apr 24;290(17):10599–609.
Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, Lasota J, Lee YS. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38(3):410–20.
Cao D, Humphrey PA, Allan RW. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer. 2009;115:2640–2651.
Andeen NK, Tretiakova MS. Metastatic Treated Malignant Germ Cell Tumors: Is SALL4 a Better Marker Than Placental Alkaline Phosphatase? Appl Immunohistochem Mol Morphol. 2016;24(3):210–4.
Park H, Lee H, Seo AN, Cho JY, Choi YR, Yoon YS, Han HS, Park YN, Kim H. SALL4 Expression in Hepatocellular Carcinomas Is Associated with EpCAM-Positivity and a Poor Prognosis. J Pathol Transl Med. 2015; 49(5):373–81.
Gautam AK, Wang C, Zeng J, Wang J, Lu J, Wei J, Huang G, Mo B, Luo M, Mo B. Expression and clinical significance of SALL4 and LGR5 in patients with lung cancer. Oncol Lett. 2015;10(6):3629–3634.
Forghanifard MM, Moghbeli M, Raeisossadati R, Tavassoli A, Mallak AJ, Boroumand- Noughabi S, Abbaszadegan MR. Role of SALL4 in the progression and metastasis of colorectal cancer. J Biomed Sci. 2013;20:6.
Hao L, Zhao Y, Wang Z, Yin H, Zhang X, He T, Song S, Sun S, Wang B, Li Z, Su Q. Expression and clinical significance of SALL4 and β-catenin in colorectal cancer. J Mol Histol. 2016;47(2):117–28.
Yang M, Xie X, Ding Y. SALL4 is a marker of poor prognosis in serous ovarian carcinoma promoting invasion and metastasis. Oncol Rep. 2016; 35(3):1796–806.
Kohashi K, Yamada Y, Hotokebuchi Y, Yamamoto H, Taguchi T, Iwamoto Y, Oda Y. ERG and SALL4 expressions in SMARCB1/INI1-deficient tumors: a useful tool for distinguishing epithelioid sarcoma from malignant rhabdoid tumor. Hum Pathol 2015;46(2):225–30.
Rodriguez E, Chen L, Ao MH, Geddes S, Gabrielson E, Askin F, Zhang H, Li QK (2014) Expression of transcript factors SALL4 and OCT4 in a subset of non-small cell lung carcinomas (NSCLC). Transl Respir Med 2(1):10.
Han SX, Wang JL, Guo XJ, He CC, Ying X, Ma JL, Zhang YY, Zhao Q, Zhu Q. Serum SALL4 is a novel prognosis biomarker with tumor recurrence and poor survival of patients in hepatocellular carcinoma. J Immunol Res. 2014:262385.
Liu J, Wang L, Yang A, Jiang P, Wang M. Up-regulation of SALL4 associated with poor prognosis in gastric cancer. Hepatogastroenterology. 2014;61(133):1459–64.
Yang JC, Chai L, Liu F, Fink LM, et al. Bmi-1 is a target gene for SALL4 in hematopoietic and leukemic cells. Proc. Natl. Acad. Sci USA. 2007;104: 10494–10499.
He J, Zhang W, Zhou Q, Zhao T, Song Y, Chai L, Li Y. Low-expression of microRNA-107 inhibits cell apoptosis in glioma by upregulation of SALL4. Int J Biochem Cell Biol. 2013;45(9):1962–73.
Cheng J, Deng R, Zhang P, Wu C, Wu K, Shi L, Liu X, Bai J, Deng M, Shuai X, Gao J, Wang G, Tao K. miR-219-5p plays a tumor suppressive role in colon cancer by targeting oncogene Sall4. Oncol Rep. 2015;34(4):1923–32.
Hupfeld T, Chapuy B, Schrader V, Beutler M, Veltkamp C, Koch R, et al. Tyrosinekinase inhibition facilitates cooperation of transcription factor SALL4 and ABC transporter A3 towards intrinsic CML cell drug resistance, Br. J. Haematol. 2013;161: 204–213.
Sugai T, Habano W, Endoh M, Konishi Y, Akasaka R, Toyota M, Yamano H, Koeda K, Wakabayashi G, Suzuki K. Molecular analysis of gastric differentiated-type intramucosal and submucosal cancers. Int J Cancer. 2010;127(11):2500–9.
Habano W, Sugai T, Jiao YF, Nakamura S. Novel approach for detecting global epigenetic alterations associated with tumor cell aneuploidy. Int J Cancer. 2007;121(7):1487–93.
Gao C, Dimitrov T, Yong KJ, Tatetsu H, Jeong HW, Luo HR, Bradner JE, Tenen DG, Chai L. Targeting transcription factor SALL4 in acute myeloid leukemia by interrupting its interaction with an epigenetic complex. Blood. 2013;121(8):1413–21.
Lu J, Jeong HW, Kong N, Yang Y, Carroll J, Luo HR, Silberstein LE, Yupoma, Chai L. Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS One. 2009; 4(5):e5577.
Yang J, Corsello TR, Ma Y. Stem cell gene SALL4 suppresses transcription through recruitment of DNA methyltransferases. J Biol Chem. 2012;287(3):1996–2005.
Ma JC, Qian J, Lin J, Qian W, Yang J, Wang CZ, Chai HY, Li Y, Chen Q, Qian Z. Aberrant hypomethylation of SALL4 gene is associated with intermediate and poor karyotypes in acute myeloid leukemia. Clin Biochem. 2013;46(4-5):304–7.
Ueno S, Lu J, He J, Li A, Zhang X, Ritz J, Silberstein LE, Chai L. Aberrant expression of SALL4 in acute B cell lymphoblastic leukemia: mechanism, function, and implication for a potential novel therapeutic target. Exp Hematol. 2014; 42(4):307-316.
Ardalan Khales S, Abbaszadegan MR, Abdollahi A, Raeisossadati R, Tousi MF, Forghanifard MM. SALL4 as a new biomarker for early colorectal cancers. J Cancer Res Clin Oncol. 2015;141(2):229-35.
Li A, Jiao Y, Yong KJ, Wang F, Gao C, Yan B, Srivastava S, Lim GS, Tang P, Yang H, Tenen DG, Chai L. SALL4 is a new target in endometrial cancer. Oncogene. 2015;34(1):63–72.
Murakami T, Yao T, Mitomi H, Morimoto T, Ueyama H, Matsumoto K, Saito T, Osada T, Nagahara A, Watanabe S. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer 2015;19(2):498–507.
Singhi AD, Seethala RR, Nason K, Foxwell TJ, Roche RL, McGrath KM, Levy RM, Luketich JD, Davison JM. Undifferentiated carcinoma of the esophagus: a clinicopathological study of 16 cases. Hum Pathol. 2015;46(3):366–75.
Forghanifard MM, Ardalan Khales S, Javdani-Mallak A, et al. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol Northwood Lond Engl. 2014;31:922.
Weissferdt A, Rodriguez-Canales J, Liu H, Fujimoto J, Wistuba II, Moran CA. Primary mediastinal seminomas: a comprehensive immunohistochemical study with a focus on novel markers. Hum Pathol. 2015;46(3):376–83.
Roma AA, Masand RP. Ovarian Brenner tumors and Walthard nests: a histologic and immunohistochemical study. Hum Pathol. 2014;45(12):2417–22.
Howitt BE, Magers MJ, Rice KR, Cole CD, Ulbright TM. Many postchemotherapy sarcomatous tumors in patients with testicular germ cell tumors are sarcomatoid yolk sac tumors: a study of 33 cases. Am J Surg Pathol. 2015;39(2):251–9.
Camparo P, Comperat EM. SALL4 is a useful marker in the diagnostic work-up of germ cell tumors in extra-testicular locations. Virchows Arch. 2013;462(3):337–41.
Liu TC, Vachharajani N, Chapman WC, Brunt EM. SALL4 immunoreactivity predicts prognosis in Western hepatocellular carcinoma patients but is a rare event: a study of 236 cases. Am J Surg Pathol. 2014;38(7):966–72.
Osada M, Aishima S, Hirahashi M, Takizawa N, Takahashi S, Nakamura K, Tanaka M, Maehara Y, Takayanagi R, Oda Y. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol. 2014;45(6):1243–50.
Masuda S, Suzuki K, Izpisua Belmonte JC. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013; 369(12):1171.
Zeng SS, Yamashita T, Kondo M, Nio K, Hayashi T, Hara Y, Nomura Y, Yoshida M, Hayashi T, Oishi N, Ikeda H, Honda M, Kaneko S. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J Hepatol 2014; 60(1):127–34.
Yoshida A, Asano N, Kawai A, Kawamoto H, Nakazawa A, Kishimoto H, Kushima R. Differential SALL4 immunoexpression in malignant rhabdoid tumours and epithelioid sarcomas. Histopathology. 2015;66(2):252–61.
Deisch J, Raisanen J, Rakheja D. Immunohistochemical expression of embryonic stem cell markers in malignant rhabdoid tumors. Pediatr Dev Pathol. 2011;14(5):353–9.
Chen Q, Qian J, Lin J, Yang J, Li Y, Wang CZ, Chai HY, Chen XX, Qian Z, Ma JC, Zhang M. Expression of SALL4 gene in patients with acute and chronic myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(2):315–9.
Bai S, Wei S, Ziober A, Yao Y, Bing Z. SALL4 and SF-1 are sensitive and specific markers for distinguishing granulosa cell tumors from yolk sac tumors.Int J Surg Pathol. 2013;21(2):121–5.
Trinh DT, Shibata K, Hirosawa T, Umezu T, Mizuno M, Kajiyama H, Kikkawa F. Diagnostic utility of CD117, CD133, SALL4, OCT4, TCL1 and glypican-3 in malignant germ cell tumors of the ovary. J Obstet Gynaecol Res. 2012;38(5):841–8.
Bai S, Wei S, Pasha TL, Yao Y, Tomaszewski JE, Bing Z. Immunohistochemical studies of metastatic germ-cell tumors in retroperitoneal dissection specimens: a sensitive and specific panel. Int J Surg Pathol. 2013;21(4):342–51.
Kilic E, Tennstedt P, Högner A, Lebok P, Sauter G, Bokemeyer C, Izbicki JR, Wilczak W. The zinc-finger transcription factor SALL4 is frequently expressed in human cancers:association with clinical outcome in squamous cell carcinoma but not in adenocarcinoma of the esophagus. Virchows Arch. 2016;468(4):483–92.
Shuai X, Zhou D, Shen T, Wu Y, Zhang J, Wang X, Li Q. Overexpression of the novel oncogene SALL4 and activation of the Wnt/beta-catenin pathway in myelodysplastic syndromes. Cancer Genet Cytogenet. 2009;194(2):119–24.
Acknowledgments
This work was supported by grants (SAG-C-DRP-100216-0040 to MA) from the Research Foundation of Marmara University (BAPKO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Dirican, E., Akkiprik, M. Functional and clinical significance of SALL4 in breast cancer. Tumor Biol. 37, 11701–11709 (2016). https://doi.org/10.1007/s13277-016-5150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5150-7