Abstract
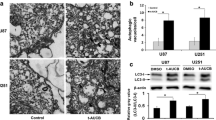
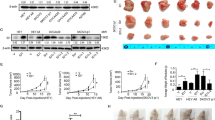
Gene associated with retinoid-interferon-induced mortality (GRIM-19), an important subunit of mitochondrial complex I, has been identified as a tumor suppressor, and its reduced expression has been reported to be associated with tumorigenesis and metastasis. Autophagy has been proposed as a protective mechanism for cell survival under various stresses, including chemotherapy. However, it remains unknown whether GRIM-19 is linked to autophagy and chemotherapy resistance. Here, we showed that suppression of GRIM-19 by shRNA enhanced cell-type-dependent autophagy by activating extracellular regulated protein kinase (ERK) and hypoxia inducible factor-1a (HIF-1a) in a reactive oxygen species (ROS)-mediated manner, and thereby conferred resistance to paclitaxel. Besides, the antioxidant N-acetyl-l-cysteine (NAC) and autophagy inhibitor 3-MA could in part overcome this resistance. We also found that GRIM-19 expression was significantly correlated with clinical stage and grade in patients with cervical cancers. Taken together, our results indicated that GRIM-19 inhibition induced autophagy and chemotherapy resistance, which could affect prognosis of cervical cancers. Our study has identified new function of GRIM-19 and its underlying mechanism, and it will provide possible avenues for therapeutic targeting in cervical cancers.






Similar content being viewed by others
References
Angell JE, Lindner DJ, Shapiro PS, Hofmann ER, Kalvakolanu DV. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J Biol Chem. 2000;275:33416–26.
Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. Embo J. 2003;22:1325–35.
Zhang J, Yang J, Roy SK, Tininini S, Hu J, Bromberg JF, et al. The cell death regulator GRIM-19 is an inhibitor of signal transducer and activator of transcription 3. Proc Natl Acad Sci U S A. 2003;100:9342–7.
He X, Cao X. Identification of alternatively spliced GRIM-19 mRNA in kidney cancer tissues. J Hum Genet. 2010;55:507–11.
Máximo V, Botelho T, Capela J, Soares P, Lima J, Taveira A, et al. Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br J Cancer. 2005;92:1892–8.
Hao M, Shu Z, Sun H, Sun R, Wang Y, Liu T, et al. GRIM-19 expression is a potent prognostic marker in colorectal cancer. Hum Pathol. 2015;46:1815–20.
Liu Q, Wang L, Wang Z, Yang Y, Tian J, Liu G, et al. GRIM-19 opposes reprogramming of glioblastoma cell metabolism via HIF1α destabilization. Carcinogenesis. 2013;34:1728–36.
Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem. 2001;276:38345–8.
Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, et al. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol. 2004;24:8447–56.
Chen Y, Yuen WH, Fu J, Huang G, Melendez AJ, Ibrahim FB, et al. The mitochondrial respiratory chain controls intracellular calcium signaling and NFAT activity essential for heart formation in Xenopus laevis. Mol Cell Biol. 2007;27:6420–32.
Chen Y, Lu H, Liu Q, Huang G, Lim CP, Zhang L, et al. Function of GRIM-19, a mitochondrial respiratory chain complex I protein, in innate immunity. J Biol Chem. 2012;287:27227–35.
Yeo WM, Isegawa Y, Chow VT. The U95 protein of human herpesvirus 6B interacts with human GRIM-19: silencing of U95 expression reduces viral load and abrogates loss of mitochondrial membrane potential. J Virol. 2008;82:1011–20.
Seo T, Lee D, Shim YS, Angell JE, Chidambaram NV, Kalvakolanu DV, et al. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death. J Virol. 2002;76:8797–807.
Barnich N, Hisamatsu T, Aguirre JE, Xavier R, Reinecker HC, Podolsky DK. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J Biol Chem. 2005;280:19021–6.
Levine B, Ranganathan R. Autophagy: snapshot of the network. Nature. 2010;466:38–40.
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19:555–66.
Huang G, Chen Y, Lu H, Cao X. Coupling mitochondrial respiratory chain to cell death: an essential role of mitochondrial complex I in the interferon-beta and retinoic acid-induced cancer cell death. Cell Death Differ. 2007;14:327–37.
Liu Y, Zhou R, Yuan X, Han N, Zhou S, Xu H, et al. DACH1 is a novel predictive and prognostic biomarker in hepatocellular carcinoma as a negative regulator of Wnt/β-catenin signaling. Oncotarget. 2015;6:8621–34.
Chu Q, Han N, Yuan X, Nie X, Wu H, Chen Y, et al. DACH1 inhibits cyclin D1 expression, cellular proliferation and tumor growth of renal cancer cells. J Hematol Oncol. 2014;7:73.
Han N, Yuan X, Wu H, Xu H, Chu Q, Guo M, et al. DACH1 inhibits lung adenocarcinoma invasion and tumor growth by repressing CXCL5 signaling. Oncotarget. 2015;6:5877–88.
Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12:465–83.
Stefanatos R, Sanz A. Mitochondrial complex I: a central regulator of the aging process. Cell Cycle. 2011;10:1528–32.
Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol Res. 2015;100:170–4.
Okon IS, Coughlan KA, Zhang M, Wang Q, Zou MH. Gefitinib-mediated reactive oxygen specie (ROS) instigates mitochondrial dysfunction and drug resistance in lung cancer cells. J Biol Chem. 2015;290:9101–10.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
He X, Zhou A, Lu H, Chen Y, Huang G, Yue X, et al. Suppression of mitochondrial complex I influences cell metastatic properties. PLoS One. 2013;8:e61677.
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838.
Yokoyama T, Kondo Y, Kondo S. Roles of mTOR and STAT3 in autophagy induced by telomere 3′ overhang-specific DNA oligonucleotides. Autophagy. 2007;3:496–8.
Peng X, Gong F, Chen Y, Jiang Y, Liu J, Yu M, et al. Autophagy promotes paclitaxel resistance of cervical cancer cells: involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis. 2014;5:e1367.
Acknowledgments
This study was funded by Wuhan Yellow Crane Medical Talent Program Grant No. 2015-12 and by the supporting program of the Ministry of Human Resource of China Oversea Returned scholars, and the National Natural Science Foundation of China (Grant No. 81572608).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Xin Yue and Peiwei Zhao have contributed equally.
Rights and permissions
About this article
Cite this article
Yue, X., Zhao, P., Wu, K. et al. GRIM-19 inhibition induced autophagy through activation of ERK and HIF-1α not STAT3 in Hela cells. Tumor Biol. 37, 9789–9796 (2016). https://doi.org/10.1007/s13277-016-4877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4877-5