Abstract
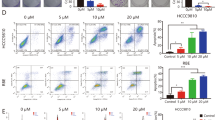
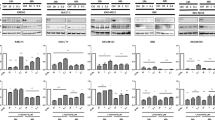
Cholangiocarcinoma (CCA) is a unique liver cancer subtype with an increasing incidence globally. The lack of specific symptoms and definite diagnostic markers results in a delayed diagnosis and disease progression. Systemic chemotherapy is commonly selected for advanced CCA even though its advantages remain unknown. Targeted therapy, especially anti-vascular endothelial growth factor (VEGF) therapy, is promising for CCA; however, improvements in the therapeutic regimen are necessary to overcome subsequent resistance. We demonstrated VEGF expression was higher in CCA cell lines than in other liver cancer cells. Secreted VEGFs played roles in the induction of peri- and intra-tumoral vascularization. VEGF neutralization by bevacizumab effectively reduced tumor growth, mainly through the suppression of angiogenesis; however, increases in the expression of hypoxia-inducible factor 1α (HIF1α) and HIF1α-responsive genes (such as VEGF, VEGFR1, VEGFR2, carbonic anhydrase (CA) IX and CAXII) indicated the potential for subsequent therapeutic resistance. Supplementation with a carbonic anhydrase inhibitor, acetazolamide, enhanced the anti-CCA effects of bevacizumab. Anti-angiogenesis and anti-proliferation were observed with the combination treatment. These results suggested a novel treatment strategy to overcome anti-angiogenesis resistance and the importance of “induced essentiality” in the treatment of CCA.






Similar content being viewed by others
References
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. doi:10.1097/SLA.0b013e318176c4d3.
Wongkham S, Silsirivanit A. State of serum markers for detection of cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):17–27.
Eckel F, Brunner T, Jelic S, Group EGW. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol / ESMO. 2010;21 Suppl 5:v65–69. doi:10.1093/annonc/mdq167.
Nagakawa T, Kayahara M, Ikeda S, Futakawa S, Kakita A, Kawarada H, et al. Biliary tract cancer treatment: results from the biliary tract cancer statistics registry in Japan. J Hepato-Biliary-Pancreat Surg. 2002;9(5):569–75. doi:10.1007/s005340200076.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi:10.1056/NEJMoa0908721.
Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99(6):862–7. doi:10.1038/sj.bjc.6604628.
Furuse J, Okusaka T. Targeted therapy for biliary tract cancer. Cancer. 2011;3(2):2243–54. doi:10.3390/cancers3022243.
Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–25. doi:10.1038/sj.bjc.6604129.
Guedj N, Zhan Q, Perigny M, Rautou PE, Degos F, Belghiti J, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009;51(1):93–101. doi:10.1016/j.jhep.2009.03.017.
Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11(1):48–54. doi:10.1016/S1470-2045(09)70333-X.
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi:10.1038/nrc2442.
Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–200. doi:10.1172/JCI70212.
Favaro E, Lord S, Harris AL, Buffa FM. Gene expression and hypoxia in breast cancer. Genome Med. 2011;3(8):55. doi:10.1186/gm271.
McIntyre A, Harris AL (2015) Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality. EMBO molecular medicine. doi:10.15252/emmm.201404271
McIntyre A, Patiar S, Wigfield S, Li JL, Ledaki I, Turley H, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 2012;18(11):3100–11. doi:10.1158/1078-0432.CCR-11-1877.
Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8(7):1867–77. doi:10.1158/1535-7163.MCT-09-0274.
Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72(7):1773–83. doi:10.1158/0008-5472.CAN-11-3831.
Kim YJ, Lee HJ, Kim TM, Eisinger-Mathason TS, Zhang AY, Schmidt B, et al. Overcoming evasive resistance from vascular endothelial growth factor a inhibition in sarcomas by genetic or pharmacologic targeting of hypoxia-inducible factor 1α. Int J Cancer. 2013;132(1):29–41. doi:10.1002/ijc.27666.
Seubwai W, Kraiklang R, Wongkham C, Wongkham S. Hypoxia enhances aggressiveness of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13(Suppl):53–8.
Thongchot S, Yongvanit P, Loilome W, Seubwai W, Phunicom K, Tassaneeyakul W, et al. High expression of HIF-1α, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev. 2014;15(14):5873–8.
Morine Y, Shimada M, Utsunomiya T, Imura S, Ikemoto T, Mori H, et al. Hypoxia inducible factor expression in intrahepatic cholangiocarcinoma. Hepato-Gastroenterology. 2011;58(110–111):1439–44. doi:10.5754/hge11156.
Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77(3):446–51. doi:10.1097/01.TP.0000110292.73873.25.
Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, et al. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11(22):3392–7.
Seubwai W, Vaeteewoottacharn K, Hiyoshi M, Suzu S, Puapairoj A, Wongkham C, et al. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting NF-kappaB. Cancer Sci. 2010;101(7):1590–5. doi:10.1111/j.1349-7006.2010.01572.x.
Gotoh K, Kariya R, Matsuda K, Hattori S, Vaeteewoottacharn K, Okada S. A novel EGFP-expressing nude mice with complete loss of lymphocytes and NK cells to study tumor-host interactions. Biosci Trends. 2014;8(4):202–5.
Goto H, Kudo E, Kariya R, Taura M, Katano H, Okada S. Targeting VEGF and interleukin-6 for controlling malignant effusion of primary effusion lymphoma. J Cancer Res Clin Oncol. 2015;141(3):465–74. doi:10.1007/s00432-014-1842-9.
Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, et al. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 2001;15(7):1215–7.
Taura M, Kariya R, Kudo E, Goto H, Iwawaki T, Amano M, et al. Comparative analysis of ER stress response into HIV protease inhibitors: lopinavir but not darunavir induces potent ER stress response via ROS/JNK pathway. Free Radic Biol Med. 2013;65:778–88. doi:10.1016/j.freeradbiomed.2013.08.161.
Taura M, Kudo E, Kariya R, Goto H, Matsuda K, Hattori S, et al. COMMD1/Murr1 reinforces HIV-1 latent infection through IκB-α stabilization. J Virol. 2015;89(5):2643–58. doi:10.1128/JVI.03105-14.
Kariya R, Matsuda K, Gotoh K, Vaeteewoottacharn K, Hattori S, Okada S. Establishment of nude mice with complete loss of lymphocytes and NK cells and application for in vivo bio-imaging. In Vivo. 2014;28(5):779–84.
Vaeteewoottacharn K, Kariya R, Matsuda K, Taura M, Wongkham C, Wongkham S, et al. Perturbation of proteasome function by bortezomib leading to ER stress-induced apoptotic cell death in cholangiocarcinoma. J Cancer Res Clin Oncol. 2013;139(9):1551–62. doi:10.1007/s00432-013-1473-6.
Mokhtari RB, Kumar S, Islam SS, Yazdanpanah M, Adeli K, Cutz E, et al. Combination of carbonic anhydrase inhibitor, acetazolamide, and sulforaphane, reduces the viability and growth of bronchial carcinoid cell lines. BMC Cancer. 2013;13:378. doi:10.1186/1471-2407-13-378.
Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, et al. Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123(4):1732–40. doi:10.1172/JCI65385.
Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24(3):349–56. doi:10.1097/MOG.0b013e3282fbf9b3.
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H, British Society of G. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69. doi:10.1136/gutjnl-2011-301748.
von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut. 2001;48(1):87–96.
McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, et al. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB Off J Int Hepato Pancreato Biliary Assoc. 2011;13(5):309–19. doi:10.1111/j.1477-2574.2010.00286.x.
Acknowledgments
We thank Mrs. I. Suzu for her technical assistance and Ms. Y. Endo for her secretarial work. This work was supported by a Grant-in-Aid for Scientific Research in Innovation Areas from the Ministry of Education, Culture, Sport Science and Technology (MEXT) of Japan (Grant No. 25460499), the Tokyo Biochemical Research Foundation, Japan (to SO and KV), the TRF Senior Research Scholar Grant to S. Wongkham (RTA5780012), and the National Research University Project of Thailand through SHeP-GMS; Khon Kaen University (to KV, NRU572012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Vaeteewoottacharn, K., Kariya, R., Dana, P. et al. Inhibition of carbonic anhydrase potentiates bevacizumab treatment in cholangiocarcinoma. Tumor Biol. 37, 9023–9035 (2016). https://doi.org/10.1007/s13277-016-4785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4785-8