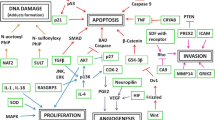
Abstract
Oral cancer incidence of 77,003 poses a major health concern in India, with 5–10 % tobacco habitués developing oral cancer. The current study examined the role of specific genomic variants in oral cancer. We examined five genomic variants represented as single nucleotide polymorphisms (SNPs) in genes associated with cell proliferation and cellular invasion. The SNPs rs2124437 (RASGRP3), rs1335022 (GRIK2), rs4512367 (PREX2), rs4748011 (CCDC3), and rs1435218 (LNX1) were analyzed in 500 histopathologically confirmed oral cancers and 500 healthy controls with a minimum of 10 years of tobacco usage. Allelic discrimination real-time PCR SYBR Green assay was used. The genotypic and allelic frequencies between cases and controls were analyzed using SPSS software (version 19) and odds ratio (OR) using Hutchon.net, indicating increased risk to oral cancers. A significant association of the SNPs in oral cancer was observed in RASGRP3 AA (rs2124437) (p < 0.000, OR 1.34, 95 % confidence interval (CI) 1.01–1.76), GRIK2 TT (rs1335022) (p = 0.008, OR 1.58, 95 % CI 1.23–2.03), PREX2 CC (p = 0.008, OR 1.56, 95 % CI 1.15–2.1), and TT (p < 0.000, OR 2.77, 1.68–4.57) genotypes, whereas the heterozygous genotypes showed higher frequencies in controls, i.e., GRIK2 CT (rs1335022) (p = 0.029, OR 0.68, 95 % CI 0.53–0.87) and PREX2 CT (p = 0.004, OR 0.49, 95 % CI 0.37–0.64), indicating protection. Coinheritance of the SNPs was associated with further increase in the risk. Thus, the SNP genotypes in the three genes, present singly or as a coinherited panel constituted “Predictive Biomarkers” indicating increased risk of oral cancer in tobacco habitués.



Similar content being viewed by others
Abbreviations
- SNP:
-
Single nucleotide polymorphisms
- WT:
-
Wild type
- HWE:
-
Hardy-Weinberg equilibrium
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- bp:
-
Base pair
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
D’Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta A. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998.
Chakrabarti S, Multani S, Dabholkar J, Saranath D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: a pilot study. Med Oncol. 2015;32(3):60.
Scheifele C, Reichart PA. Is there a natural limit of the transformation rate of oral leukoplakia. Oral Oncol. 2003;39(5):470–5.
Bhatnagar R, Dabholkar J, Saranath D. Genome-wide disease association study in chewing tobacco associated oral cancers. Oral Oncol. 2012;48(9):831–5.
Chien MH, Yang JS, Chu YH, Lin CH, Wei LH, Yang SF, et al. Impacts of CA9 gene polymorphisms and environmental factors on oral-cancer susceptibility and clinicopathologic characteristics in Taiwan. PLoS One. 2012;7(12):5–12.
Ignatova Z, Martínez-Pérez I, Zimmermann K-H. DNA computing models. Springer Science & Business Media, 2008; 288.
Satagopan JM, Verbel DA, Venkatraman ES, Offit KE, Colin B, Verbel DA, et al. Two-stage designs for gene-disease association studies. 2015;58(1):163–70.
Pilato B, Martinucci M, Danza K, Pinto R, Petriella D, Lacalamita R, et al. Mutations and polymorphic BRCA variants transmission in breast cancer familial members. Breast Cancer Res Treat. 2011;125(3):651–7.
Erichsen HC, Chanock SJ. SNPs in cancer research and treatment. Br J Cancer. 2004;90(4):747–51.
Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4):a006098.
Easton DF, Eeles R a. Genome-wide association studies in cancer. Hum Mol Genet. 2008;17(R2):R109–15.
Yang D, Tao J, Li L, Kedei N, Tóth ZE, Czap A, et al. RasGRP3, a Ras activator, contributes to signaling and the tumorigenic phenotype in human melanoma. Oncogene. 2011;30(45):4590–600.
Rosenfeldt H, Vázquez-Prado J, Gutkind JS. P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 2004;572(1–3):167–71.
Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal. 2012;24(2):353–62.
Wu CS, Lu YJ, Li HP, Hsueh C, Lu CY, Leu YW, et al. Glutamate receptor, ionotropic, kainate 2 silencing by DNA hypermethylation possesses tumor suppressor function in gastric cancer. Int J Cancer. 2010;126(11):2542–52.
Kim MS, Yamashita K, Baek JH, Park HL, Carvalho AL, Osada M, et al. N-methyl-d-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res. 2006;66(7):3409–18.
Zheng D, Sun Y, Gu S, Ji C, Zhao W, Xie Y, et al. LNX (ligand of Numb-protein X) interacts with RhoC, both of which regulate AP-1-mediated transcriptional activation. Mol Biol Rep. 2010;37(5):2431–7.
Azad AK, Chakrabarti S, Xu Z, Davidge ST, Fu Y. Coiled-coil domain containing 3 (CCDC3) represses tumor necrosis factor-α/nuclear factor κB-induced endothelial inflammation. Cell Signal. 2014;26(12):2793–800.
Xavier S, Elisabet G, Joan V, Raquel I, and Victor M. Bioinformatics. 2006; 22: 1928–1929.
Bland M, Altman D. Statistics notes: the odds ratio. BMJ. 2000;320:1468.
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4(2):45–61.
Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485(7399):502–6.
Salanti G, Amountza G, Ntzani EE, Ioannidis JP a. Hardy-Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur J Hum Genet. 2005;13(7):840–8.
National Center for biotechnology information. http://www.ncbi.nlm.nih.gov/snp (Accessed 26th September, 2015)
Yang D, Kedei N, Li L, Tao J, Velasquez JF, Michalowski AM, et al. RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 2010;70(20):7905–17.
Komkov AY, Maschan MA, Shvets VI, Lebedev YB. Functional analysis of polymorphic insertions of Alu retroelements in acute lymphoblastic leukemia patients. Russ J Bioorganic Chem. 2012;38(3):306–18.
Li M, Gardiner JC, Breslau N, Anthony JC, Lu Q. A non-parametric approach for detecting gene-gene interactions associated with age-at-onset outcomes. BMC Genet. 2014;15(1):79.
Suzuki A, Mimaki S, Yamane Y, Kawase A, Matsushima K, Suzuki M, et al. Identification and characterization of cancer mutations in Japanese lung adenocarcinoma without sequencing of normal tissue counterparts. PLoS One. 2013;8(9):1–11.
Tsai M-H, Chen W-C, Tsai C-H, Hang L-W, Tsai F-J. Interleukin-4 gene, but not the interleukin-1 beta gene polymorphism, is associated with oral cancer. J Clin Lab Anal. 2005;19(3):93–8.
Vairaktaris E, Yannopoulos A, Vassiliou S, Serefoglou Z, Vylliotis A, Nkenke E, et al. Strong association of interleukin-4 (−590 C/T) polymorphism with increased risk for oral squamous cell carcinoma in Europeans. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;104(6):796–802.
Yang L, Zhu X, Liang X, Ling Z, Li R. Association of IL-8-251A > T polymorphisms with oral cancer risk: evidences from a meta-analysis. Tumor Biol. 2014;35(9):9211–8.
Gaur P, Mittal M, Mohanti BK, Das SN. Functional genetic variants of TGF-β1 and risk of tobacco-related oral carcinoma in high-risk Asian Indians. Oral Oncol. 2011;47(12):1117–21.
Hsu H, Yang Y, Shieh T, Chen C. TGF-β1 and IL-10 single nucleotide polymorphisms as risk factors for oral cancer in Taiwanese. Kaohsiung J Med Sci. 2014;100:1–7.
Lin CW, Chuang CY, Tang CH, Chang JL, Lee LM, Lee WJ, et al. Combined effects of ICAM-1 single-nucleotide polymorphisms and environmental carcinogens on oral cancer susceptibility and clinicopathologic development. PLoS One. 2013;8(9):1–8.
Jha R, Gaur P, Sharma SC, Das SN. Single nucleotide polymorphism in hMLH1 promoter and risk of tobacco-related oral carcinoma in high-risk Asian Indians. Gene. 2013;526(2):223–7.
Anantharaman D, Chaubal PM, Kannan S, Bhisey R a, Mahimkar MB. Susceptibility to oral cancer by genetic polymorphisms at CYP1A1, GSTM1 and GSTT1 loci among Indians: tobacco exposure as a risk modulator. Carcinogenesis. 2007;28(7):1455–62.
Shukla D, Kale AD, Hallikerimath S, Vivekanandhan S, Venkatakanthaiah Y. Genetic polymorphism of drug metabolizing enzymes (GSTM1 and CYP1A1) as risk factors for oral premalignant lesions and oral cancer. Biomed Pap. 2012;156(3):253–9.
Masood N, Kayani MA, Malik FA, Mahjabeen I, Baig RM, Faryal R. Genetic variation in carcinogen metabolizing genes associated with oral cancer in Pakistani population. Asian Pac J Cancer Prev. 2011;12(2):491–5.
Singh R, Haridas N, Shah F, Patel J, Shukla S, Patel P. Gene polymorphisms, tobacco exposure and oral cancer susceptibility: a study from Gujarat, West India. Oral Dis. 2014;20(1):84–93.
Acknowledgments
The authors gratefully acknowledge the support of Cancer Patients Aids Association (CPAA), Foundation of Medical Research (FMR), and Balabai Nanavati Hospital, Mumbai, India, for the project.
Role of the funding source
Funding was provided by the affiliated university for procurement of reagents for the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Ethics approval and consent to participate
The study was approved by the Institute Ethics Committees of NMIMS (deemed-to-be) University, Mumbai; Cancer Patients Aid Association, Mumbai; and Prince Aly Khan Hospital, Mumbai. All subjects gave written informed consent for voluntary participation in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Primer sequences for allelic discrimination PCR and sequencing primers. (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Multani, S., Pradhan, S. & Saranath, D. Gene polymorphisms and oral cancer risk in tobacco habitués. Tumor Biol. 37, 6169–6176 (2016). https://doi.org/10.1007/s13277-015-4448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4448-1