Abstract
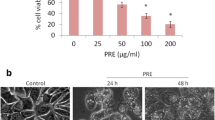
Esculetin (6,7-dihydroxycoumarin), a derivative of coumarin compound, is found in traditional medicinal herbs. It has been shown that esculetin triggers diverse cellular signal transduction pathways leading to regulation of physiology in different models. However, whether esculetin affects Ca2+ homeostasis in breast cancer cells has not been explored. This study examined the underlying mechanism of cytotoxicity induced by esculetin and established the relationship between Ca2+ signaling and cytotoxicity in human breast cancer cells. The results showed that esculetin induced concentration-dependent rises in the intracellular Ca2+ concentration ([Ca2+]i) in ZR-75-1 (but not in MCF-7 and MDA-MB-231) human breast cancer cells. In ZR-75-1 cells, this Ca2+ signal response was reduced by removing extracellular Ca2+ and was inhibited by the store-operated Ca2+ channel blocker 2-aminoethoxydiphenyl borate (2-APB). In Ca2+-free medium, pre-treatment with the endoplasmic reticulum Ca2+ pump inhibitor thapsigargin (TG) abolished esculetin-induced [Ca2+]i rises. Conversely, incubation with esculetin abolished TG-induced [Ca2+]i rises. Esculetin induced cytotoxicity that involved apoptosis, as supported by the reduction of mitochondrial membrane potential and the release of cytochrome c and the proteolytic activation of caspase-9/caspase-3, which were partially reversed by pre-chelating cytosolic Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM). Moreover, esculetin increased the percentage of cells in G2/M phase and regulated the expressions of p53, p21, CDK1, and cyclin B1. Together, in ZR-75-1 cells, esculetin induced [Ca2+]i rises by releasing Ca2+ from the ER and causing Ca2+ influx through 2-APB-sensitive store-operated Ca2+ entry. Furthermore, esculetin activated Ca2+-associated mitochondrial apoptotic pathways that involved G2/M cell cycle arrest.

The summary of esculetin-evoked [Ca2+]i rises and -activated Ca2+-associated mitochondrial apoptotic pathways that involved cell cycle arrest. The natural coumarin derivative esculetin caused Ca2+ influx via 2-APB-sensitive store-operated Ca2+ entry and induced Ca2+ release from the endoplasmic reticulum. Moreover, esculetin activated the mitochondrial pathway of apoptosis in a Ca2+-associated manner that involved G2/M arrest.









Similar content being viewed by others
References
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62.
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22.
Moghadamtousi SZ, Goh BH, Chan CK, Shabab T, Kadir HA. Bio-logical activities and phytochemicals of Swietenia macrophylla King. Molecules. 2013;18:10465–83.
Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421.
Khan MK, Zill EH, Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J Food Compos Anal. 2014;33:85–104.
Chang WS, Lin CC, Chuang SC, Chiang HC. Superoxide anion scavenging effect of coumarins. Am J Chin Med. 1996;24:11–7.
Tubaro A, Del Negro P, Ragazzi E, Zampiron S, Della LR. Anti-inflammatory and peripheral analgesic activity of esculetin in vivo. Pharmacol Res Commun. 1988;20:83–5.
Egan D, O'Kennedy R, Moran E, Cox D, Prosser E, Thornes RD. The pharmacology, metabolism, analysis, and applications of coumarin and coumarin-related compounds. Drug Metab Rev. 1990;22:503–29.
Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr. 2004;44:575–86.
Lacy A, O'Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10:3797–811.
Wang CJ, Hsieh YJ, Chu CY, Lin YL, Tseng TH. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002;183:163–8.
Kok SH, Yeh CC, Chen ML, Kuo MY. Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation in human oral cancer SAS cells. Oral Oncol. 2009;45:1067–72.
Rubio V, Calviño E, García-Pérez A, Herráez A, Diez JC. Human acute promyelocytic leukemia NB4 cells are sensitive to esculetin through induction of an apoptotic mechanism. Chem Biol Interact. 2014;220:129–39.
Kim AD, Han X, Piao MJ, Hewage SR, Hyun CL, Cho SJ, et al. Esculetin induces death of human colon cancer cells via the reactive oxygen species-mediated mitochondrial apoptosis pathway. Environ Toxicol Pharmacol. 2015;39:982–9.
Earashi M, Noguchi M, Tanaka M. In vitro effects of eicosanoid synthesis inhibitors in the presence of linoleic acid on MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 1996;37:29–37.
Clapham DE. Calcium signaling. Cell. 1995;80:259–68.
Clapham DE. Calcium signaling. Cell. 2007;131:1047–58.
Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67:1–21.
Putney Jr JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12.
Tang S, Wang X, Shen Q, Yang X, Yu C, Cai C, et al. Mitochondrial Ca2+ uniporter is critical for store-operated Ca2+ entry-dependent breast cancer cell migration. Biochem Biophys Res Commun. 2015;458:186–93.
Bootman M. Intracellular calcium. Questions about quantal Ca2+ release. Curr Biol. 1994;4:169–72.
Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309.
Azimi I, Roberts-Thomson SJ, Monteith GR. Calcium influx pathways in breast cancer: opportunities for pharmacological intervention. Br J Pharmacol. 2014;171:945–60.
Evan GI, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr Opin Cell Biol. 1995;7:825–34.
King KL, Cidlowsky JA. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995;58:175–80.
Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–84.
Golstein P. Controlling cell death. Science. 1997;275:1081–2.
Bold RJ, Ishizuka J, Yao CZ, Townsend Jr CM, Thompson JC. Bombesin stimulates in vitro growth of human breast cancer independent of estrogen receptors status. Anticancer Res. 1998;18:4051–6.
Chang HT, Huang JK, Wang JL, Cheng JS, Lee KC, Lo YK, et al. Tamoxifen-induced increases in cytoplasmic free Ca2+ levels in human breast cancer cells. Breast Cancer Res Treat. 2002;71:125–31.
Chang HT, Chou CT, Kuo DH, Shieh P, Jan CR, Liang WZ. The mechanism of Ca2+ movement in the involvement of baicalein-induced cytotoxicity in ZR-75-1 human breast cancer cells. J Nat Prod. 2015;78:1624–34.
Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50.
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9.
Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013;126:1260–7.
Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–83.
Yoon MJ, Lee AR, Jeong SA, Kim YS, Kim JY, Kwon YJ, et al. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget. 2014;5:6816–31.
Kumar B, Kumar A, Ghosh S, Pandey BN, Mishra KP, Hazra B. Diospyrin derivative, an anticancer quinonoid, regulates apoptosis at endoplasmic reticulum as well as mitochondria by modulating cytosolic calcium in human breast carcinoma cells. Biochem Biophys Res Commun. 2012;417:903–9.
Noguchi M, Earashi M, Minami M, Miyazaki I, Tanaka M, Sasaki T. Effects of piroxicam and esculetin on the MDA-MB-231 human breast cancer cell line. Prostaglandins Leukot Essent Fat Acids. 1995;53:325–9.
Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–404.
Poulin R, Lessard M, Zhao C. Inorganic cation dependence of putrescine and spermidine transport in human breast cancer cells. J Biol Chem. 1995;270:1695–704.
Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6.
O’Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303.
Lee KC, Chou KJ, Cheng JS, Wang JL, Tang KY, Tseng LL, et al. Novel effects of 5,8,11-eicosatriynoic acid, a lipoxygenase inhibitor, on Ca2+ mobilization in Madin Darby canine kidney cells. Pharmacol Toxicol. 2001;88:20–6.
Yamamura H, Nagano N, Hirano M, Muraki K, Watanabe M, Imaizumi Y. Activation of Ca2+-dependent K+ current by nordihydroguaiaretic acid in porcine coronary arterial smooth muscle cells. J Pharmacol Exp Ther. 1999;291:140–6.
Lee KL, Dai Q, Hansen EL, Saner CN, Price TM. Modulation of ATP-induced calcium signaling by progesterone in T47D-Y breast cancer cells. Mol Cell Endocrinol. 2010;319:109–15.
Le Bihan S, Marsaud V, Mercier-Bodard C, Baulieu EE, Mader S, White JH, et al. Calcium/calmodulin kinase inhibitors and immunosuppressant macrolides rapamycin and FK506 inhibit progestin- and glucocorticosteroid receptor-mediated transcription in human breast cancer T47D cells. Mol Endocrinol. 1998;12:986–1001.
McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10:448–60.
Parekh AB, Putney Jr JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810.
Michelangeli F, East JM. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans. 2011;39:789–97.
Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL, Hou LH, et al. ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chem Biol Interact. 2011;190:16–27.
Tyagi M, Patro BS, Chattopadhyay S. Mechanism of the malabaricone C-induced toxicity to the MCF-7 cell line. Free Radic Res. 2014;48:466–77.
Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9.
Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 1777;2008:808–16.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16.
Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995;92:8493–7.
Guillouf C, Rosselli F, Krishnaraju K, Moustacchi E, Hoffman B, Liebermann DA. p53 involvement in control of G2 exit of the cell cycle: role in DNA damage-induced apoptosis. Oncogene. 1995;10:2263–70.
Matsunaga K, Yoshimi N, Yamada Y, Shimizu M, Kawabata K, Ozawa Y, et al. Inhibitory effects of nabumetone, a cyclooxygenase-2 inhibitor, and esculetin, a lipoxygenase inhibitor, on N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Jpn J Cancer Res. 1998;89:496–501.
Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM, Tseng TH. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch Toxicol. 2000;74:467–72.
Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, et al. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–30.
Yamada H, Watanabe K, Saito T, Hayashi H, Niitani Y, Kikuchi T, et al. Esculetin (dihydroxycoumarin) inhibits the production of matrix metalloproteinases in cartilage explants, and oral administration of its prodrug, CPA-926, suppresses cartilage destruction in rabbit experimental osteoarthritis. J Rheumatol. 1999;26:654–62.
Kim JS, Ha TY, Ahn J, Kim S. Analysis and distribution of esculetin in plasma and tissues of rats after oral administration. Prev Nutr Food Sci. 2014;19:321–6.
Acknowledgments
This work was supported by grants from Kaohsiung Veterans General Hospital (VGHKS104-116) to Chung-Ren Jan and Ministry of Science and Technology (MOST103-2314-B-075B-002) to Hong-Tai Chang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Chang, HT., Chou, CT., Lin, YS. et al. Esculetin, a natural coumarin compound, evokes Ca2+ movement and activation of Ca2+-associated mitochondrial apoptotic pathways that involved cell cycle arrest in ZR-75-1 human breast cancer cells. Tumor Biol. 37, 4665–4678 (2016). https://doi.org/10.1007/s13277-015-4286-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4286-1