Abstract
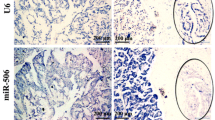
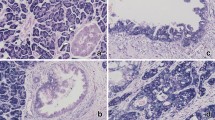
MiR-483-3p has been reported to be widely involved in diverse human malignancies. However, the exact role of miR-483-3p remains elusive in pancreatic ductal adenocarcinoma (PDAC). The objective of this study is to determine the expression pattern and clinical implications of miR-483-3p in PDAC. MiR-483-3p levels were evaluated by locked nucleic acid-in situ hybridization (LNA-ISH) in a tissue microarray including 63 PDAC tumors and 10 normal pancreatic tissues, followed by evaluation in an independent set of 117 pairs of matched PDAC tumors and adjacent tumor-free pancreatic tissues. Expression of miR-483-3p was further evaluated in pancreatic intra-epithelial neoplasias (PanINs) and chronic pancreatitis (CP). The impact of miR-483-3p on cell proliferation, growth, and anchorage-independent colony formation was also assessed in vitro and in vivo. Microarray analysis revealed that miR-483-3p was positively stained in 61 (96.8 %) PDAC samples, but not detectable in normal pancreatic duct tissue. In the 117 PDAC samples, 100 % were miR-483-3p positive, with 55.6 % (65/117) strongly positive, compared to only 13.7 % (16/117) weakly positive in adjacent normal pancreatic duct tissues. MiR-483-3p expression was associated with tumor grading (p < 0.05) and was an independent predictor of poor overall survival in multivariate analysis (HR = 2.584; 95 % CI = 1.268–5.264). Moreover, from PanIN1 to PanIN3, the rate of strong miR-483-3p-positive staining was 0 % (0/39), 14.8 % (4/27), and 87.5 % (14/16), respectively. Six (54.5 %) CP samples were only weakly stained for miR-483-3p. Inhibition of miR-483-3p suppressed cell proliferation, growth, and colony formation in vitro and decreased tumor cell growth in nude mouse xenografts in vivo. These results suggest that aberrant miR-483-3p expression is an early event in PDAC tumorigenesis and is associated with tumor differentiation and prognosis. It also may be a potential target for PDAC molecular therapeutics.





Similar content being viewed by others
References
Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–44.
He Y, Zheng R, Li D, et al. Pancreatic cancer incidence and mortality patterns in China, 2011. Chin J Cancer Res. 2015;27:29–37.
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79.
Pisters PW, Wolff RA, Crane CH, Evans DB. Combined-modality treatment for operable pancreatic adenocarcinoma. Oncology (Williston Park). 2005;19:393–404. 409–10,412-6.
Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60.
Ross JS, Carlson JA, Brock G. miRNA: the new gene silencer. Am J Clin Pathol. 2007;128:830–6.
Law PT, Wong N. Emerging roles of microRNA in the intracellular signaling networks of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:437–49.
Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69.
Otsuka M, Kishikawa T, Yoshikawa T, et al. The role of microRNAs in hepatocarcinogenesis: current knowledge and future prospects. J Gastroenterol. 2014;49:173–84.
Wang V, Wu W. MicroRNA-based therapeutics for cancer. Bio Drug. 2009;23:15–23.
Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8.
Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2:e610.
Joyce CE, Zhou X, Xia J, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet. 2011;20:4025–40.
Kong D, Piao YS, Yamashita S, et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–60.
Wang W, Zhao LJ, Tan YX, Ren H, Qi ZT. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis. 2012;33:1113–20.
Wang W, Zhao LJ, Tan YX, Ren H, Qi ZT. Identification of deregulated miRNAs and their targets in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol. 2012;18:5442–53.
Yi C, Wang Q, Wang L, et al. MiR-663, a microRNA targeting p21 (WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene. 2012;31:4421–33.
Veronese A, Lupini L, Consiglio J, et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010;70:3140–9.
Wang C, Sun Y, Wu H, et al. Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers. Histopathology. 2014;64:567–76.
Tang W, Zhu J, Su S, et al. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One. 2012;7:e51702.
Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36.
Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–6.
Hao J, Zhang S, Zhou Y, Hua X, Shao C. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Lett. 2011;585:207–13.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49.
Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224–32.
Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–12.
Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–92.
Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin N Am. 2007;36:831–49.
Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–51.
Ekbom A, McLaughlin JK, Karlsson BM, et al. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625–7.
Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–52.
Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer: International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7.
Yan L, McFaul C, Howes N, et al. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology. 2005;128:2124–30.
Logsdon CD, Ji B. Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:S40–3.
Lee CS, Rush M, Charalambous D, Rode J. Immunohistochemical demonstration of the p53 tumour suppressor gene product in cancer of the pancreas and chronic pancreatitis. J Gastroenterol Hepatol. 1993;8:465–9.
Özata DM, Caramuta S, Velázquez-Fernández D, et al. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr Relat Cancer. 2011;18:643–55.
Bertero T, Gastaldi C, Bourget-Ponzio I, et al. miR-483-3p controls proliferation in wounded epithelial cells. FASEB J. 2011;25:3092–105.
Bertero T, Gastaldi C, Bourget-Ponzio I, et al. CDC25A targeting by miR-483-3p decreases CCND–CDK4/6 assembly and contributes to cell cycle arrest. Cell Death Differ. 2013;20:800–11.
Bertero T, Bourget-Ponzio I, Puissant A, et al. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle. 2013;12:2183–93.
Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8.
Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810.
Acknowledgments
This study was supported by The National Nature Science Foundation of China (No. 30973470, No. 81172334, and No. 81400664).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Sun, Y., Wu, H. et al. Elevated miR-483-3p expression is an early event and indicates poor prognosis in pancreatic ductal adenocarcinoma. Tumor Biol. 36, 9447–9456 (2015). https://doi.org/10.1007/s13277-015-3690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3690-x