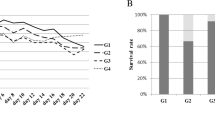
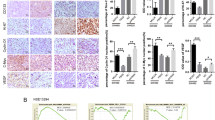
Abstract
Removal of primary tumors often leads to increases in growth of metastatic tumor cells. Thus, development of an efficient treatment to inhibit the growth of metastatic tumor cells after resection of primary tumors appears to be critical for cancer therapy. Here, we reported that administration of a Chinese medicine Shiquandabutao (SQDBT) after removal of the primary cancer significantly inhibited the growth of metastatic cancer cells in mouse liver. Further analyses showed that the effect of SQDBT resulted from one of its main component, Siwutang (SWT), rather than from another main component, Sijunzitang (SJZT). Moreover, we found that the soluble Flt-1 from SWT neutralized the increased placental growth factor (PLGF) secreted by the metastatic cancer cells after primary cancer resection and subsequently inhibited the cancer neovascularization to suppress the metastatic cancer growth. Thus, our study reveals an essential role of SQDBT in inhibiting the growth of metastatic cancer after removal of primary cancer and further highlights PLGF as a potential target for metastatic cancer treatment.








Similar content being viewed by others
References
Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–8.
Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the achilles heel of cancer surgery. Ann Surg Oncol. 2011;18:603–7.
Curcio LD, Chu DZ, Ahn C, Williams Jr WL, Paz IB, Riihimaki D, et al. Local recurrence in breast cancer: implications for systemic disease. Ann Surg Oncol. 1997;4:24–7.
Kosari K, Gomes M, Hunter D, Hess DJ, Greeno E, Sielaff TD. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg. 2002;6:255–63.
Scott AD, Crane P, Staunton MD. Chondrosarcoma—local recurrence and systemic embolization. J R Soc Med. 1990;83:48–9.
Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–91.
Eichmann A, Simons M. Vegf signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–93.
Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, et al. Placenta growth factor-1 antagonizes vegf-induced angiogenesis and tumor growth by the formation of functionally inactive plgf-1/vegf heterodimers. Cancer Cell. 2002;1:99–108.
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-plgf inhibits growth of vegf(r)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75.
Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of plgf in the intra- and intermolecular cross talk between the vegf receptors flt1 and flk1. Nat Med. 2003;9:936–43.
Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J, et al. Pancreatic duct cells as a source of vegf in mice. Diabetologia. 2014;57:991–1000.
Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, et al. Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288:8636–46.
Yokoyama Y, Dhanabal M, Griffioen AW, Sukhatme VP, Ramakrishnan S. Synergy between angiostatin and endostatin: Inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–6.
Indraccolo S, Minuzzo S, Gola E, Habeler W, Carrozzino F, Noonan D, et al. Generation of expression plasmids for angiostatin, endostatin and timp-2 for cancer gene therapy. Int J Biol Markers. 1999;14:251–6.
Harris AL. Are angiostatin and endostatin cures for cancer? Lancet. 1998;351:1598–9.
Huang SM, Chien LY, Tai CJ, Chiou JF, Chen CS, Tai CJ. Effectiveness of 3-week intervention of shi quan da bu tang for alleviating hematotoxicity among patients with breast carcinoma receiving chemotherapy. Integr Cancer Ther. 2013;12:136–44.
Mita Y, Dobashi K, Shimizu Y, Nakazawa T, Mori M. Surface expression of toll-like receptor 4 on thp-1 cells is modulated by bu-zhong-yi-qi-tang and shi-quan-da-bu-tang. Methods Find Exp Clin Pharmacol. 2002;24:67–70.
Wu Y, Zhang Y, Wu JA, Lowell T, Gu M, Yuan CS. Effects of erkang, a modified formulation of chinese folk medicine shi-quan-da-bu-tang, on mice. J Ethnopharmacol. 1998;61:153–9.
Tatsuta M, Iishi H, Baba M, Nakaizumi A, Uehara H. Inhibition by shi-quan-da-bu-tang (tj-48) of experimental hepatocarcinogenesis induced by n-nitrosomorpholine in Sprague–Dawley rats. Eur J Cancer. 1994;30A:74–8.
Zee-Cheng RK. Shi-quan-da-bu-tang (ten significant tonic decoction), sqt. A potent chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find Exp Clin Pharmacol. 1992;14:725–36.
Sakagami Y, Mizoguchi Y, Miyajima K, Kuboi H, Kobayashi K, Kioka K, et al. Antitumor activity of shi-quan-da-bu-tang and its effects on interferon-gamma and interleukin 2 production. Arerugi = [Allergy]. 1988;37:57–60.
Nishiuchi T, Okutani Y, Yamagishi Y, Fujita T, Imataki O, Ohnishi H, et al. Synergistic effect between juzen-taiho-to, a japanese traditional herbal medicine, and gemcitabine single-agent chemotherapy for advanced biliary tract cancer. J Altern Complement Med. 2013;19:593–7.
Sakamoto S, Kudo H, Kuwa K, Suzuki S, Kato T, Kawasaki T, et al. Anticancer effects of a chinese herbal medicine, juzen-taiho-to, in combination with or without 5-fluorouracil derivative on DNA-synthesizing enzymes in 1,2-dimethylhydrazine induced colonic cancer in rats. Am J Chinese Med. 1991;19:233–41.
Saiki I. A kampo medicine “juzen-taiho-to”–prevention of malignant progression and metastasis of tumor cells and the mechanism of action. Biol Pharm Bull. 2000;23:677–88.
Lian Z, Niwa K, Gao J, Tagami K, Hashimoto M, Yokoyama Y, et al. Shimotsu-to is the agent in juzen-taiho-to responsible for the prevention of endometrial carcinogenesis in mice. Cancer Lett. 2002;182:19–26.
Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer. 1999;79:1392–8.
Acknowledgments
This work was financially supported by National Nature Science Foundation of China (no. 81273733).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Zhang, Y., Li, A., Peng, W. et al. RETRACTED ARTICLE: Efficient inhibition of growth of metastatic cancer cells after resection of primary colorectal cancer by soluble Flt-1. Tumor Biol. 36, 7399–7407 (2015). https://doi.org/10.1007/s13277-015-3434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3434-y