Abstract
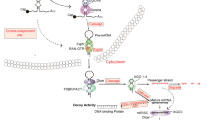
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expressions at posttranscriptional level. Growing evidence points to their significant role in the acquisition of drug resistance in cancers. Studies show that miRNAs are often aberrantly expressed in human cancer cells which are associated with tumorigenesis, metastasis, invasiveness, and drug resistance. Breast cancer is the leading cause of cancer-induced death in women. Over the last decades, increasing attention has been paid to the effects of miRNAs on the development of breast cancer drug resistance. Among them, miR-155 takes part in a sequence of bioprocesses that contribute to the development of such drug resistance, including repression of FOXO3a, enhancement of epithelial-to-mesenchymal transition (EMT) and mitogen-activated protein kinase (MAPK) signaling, reduction of RhoA, and affecting the length of telomeres. In this review, we discuss the role of miR-155 in the acquisition of breast cancer drug resistance. This will provide a new way in antiresistance treatment of drug-resistant breast cancer.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Pasquier J, Magal P, Boulange-Lecomte C, Webb G, Le Foll F. Consequences of cell-to-cell P-glycoprotein transfer on acquired multidrug resistance in breast cancer: a cell population dynamics model. Biol Direct. 2011;6:5.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Tang R, Li L, Zhu D, Hou D, Cao T, Gu H. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell Res. 2012;22:504–15.
Kutanzi KR, Yurchenko OV, Beland FA, Checkhun VF, Pogribny IP. MicroRNA-mediated drug resistance in breast cancer. Clin Epigenetics. 2011;2:171–85.
Teng G, Papavasiliou FN. Shhh! Silencing by microRNA-155. Philos Trans R Soc Lond B Biol Sci. 2009;364:631–7.
Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, et al. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228.
Cui X, Guo Y, Yao H. Analysis of microRNA in drug-resistant breast cancer cell line MCF-7/ADR. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1813–5.
Clurman BE, Hayward WS. Multiple proto-oncogene activations in avian leukosis virus-induced lymphomas: evidence for stage-specific events. Mol Cell Biol. 1989;9:2657–64.
Sandhu SK, Volinia S, Costinean S, Galasso M, Neinast R, Santhanam R, et al. MiR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Emu-miR-155 transgenic mouse model. Proc Natl Acad Sci U S A. 2012;109:20047–52.
Zeng H, Fang C, Nam S, Cai Q, Long X. The clinicopathological significance of MicroRNA-155 in breast cancer: a meta-analysis. Biomed Res Int. 2014;2014:1–7.
Zhang JLQM. Analysis of miR-205 and miR-155 expression in the blood of breast cancer patients. Chin J Cancer Res. 2013;25:46–54.
Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomark. 2012;21:1236–43.
Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2013;32:2992–3000.
Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, et al. MicroRNA-155 is regulated by the transforming growth factor /Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–84.
Johansson J, Berg T, Kurzejamska E. MiR-155-mediated loss of C/EBPb shifts the TGF-b response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene 2013:5614–5624.
Martin EC, Krebs AE, Burks HE, Elliott S, Baddoo M, Collins-Burow BM, et al. MiR-155 induced transcriptome changes in the MCF-7 breast cancer cell line leads to enhanced mitogen activated protein kinase signaling. Genes Cancer. 2014;5:353–64.
Shu-Rong Zheng GGQZ. Effects of miR-155 antisense oligonucleotide on breast carcinoma cell line MDA-MB-157 and implanted tumors. Asian Pac J Cancer Prev. 2013;4:2361–6.
Nho RS. FoxO3a and disease progression. World J Biol Chem. 2014;5:346.
Monsalve M, Olmos Y. The complex biology of FOXO. Curr Drug Targets. 2011;12:1322–50.
Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87.
Kong W, He L, Coppola M, Guo J, Esposito NN, Coppola DCJ. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–79.
Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang HT, et al. MicroRNA-155 regulates cell proliferation and invasion by targeting FOXO3a in glioma. Oncol Rep. 2013;30:2111–8.
Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol. 2000;20:9138–48.
Grabinski N, Möllmann K, Milde-Langosch K, Müller V, Schumacher U, Brandt B, et al. AKT3 regulates ErbB2, ErbB3 and estrogen receptor α expression and contributes to endocrine therapy resistance of ErbB2+ breast tumor cells from Balb-neuT mice. Cell Signal. 2014;26:1021–9.
Brown I, Shalli K, McDonald SL, Moir SE, Hutcheon AW, Heys SD, et al. Reduced expression of p27 is a novel mechanism of docetaxel resistance in breast cancer cells. Breast Cancer Res. 2004;6:R601–7.
Raina D, Uchida Y, Kharbanda A, Rajabi H, Panchamoorthy G, Jin C, et al. Targeting the MUC1-C oncoprotein downregulates HER2 activation and abrogates trastuzumab resistance in breast cancer cells. Oncogene. 2014;33:3422–31.
Liam O, Connor AS, Lorraine A, O Reilly GH, Jerry M, Adams SCA, Huang DCS. Bim bang in cell death O’Connor L, Strasser A, O’Reilly A et al. (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis EMBO J. 17, 384–395. Immunology Today 1998;19:99.
Sonenshein SGAG. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004;24:8681–90.
Shaocong Guo YLQT. DEF1 down-regulates ER—a expression and confers tamoxifen resistance in breast cancer. PLoS ONE. 2012;12:e52380.
Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–65.
Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–25.
Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, DiStefano PS, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4.
Park S, Guo J, Kim D, Cheng JQ. Identification of 24p3 as a direct target of Foxo3a regulated by interleukin-3 through the phosphoinositide 3-kinase/Akt pathway. J Biol Chem. 2009;284:2187–93.
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73.
Lee JM. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81.
Johansson J, Berg T, Kurzejamska E. MiR-155-mediated loss of C/EBPb shifts the TGF-b response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer 2013; 5614–5624.
Wang J, Wu J. Role of miR-155 in breast cancer. Front Biosci (Landmark Ed). 2012;17:2350–5.
Qu H, Fang L, Duan L, Long X. Expression of ABCG2 and p-glycoprotein in residual breast cancer tissue after chemotherapy and their correlation with epithelial-mesenchymal transition. Zhonghua Bing Li Xue Za Zhi. 2014;43:236–40.
Li W, Liu C, Wang H. Highexpression of Snail leads to the P-gp modulateds MDR in breast cancer cell MCF-7. Chin Pharmacol Bull. 2010:87–90.
Tang Y, Wang H, Chen W. Epithelial-mesenchymal transition modulates P-glucoprotein-induced multidrug resistance in breast cancer MCF-7 cells via p38-MAPK. China J Cancer Biother. 2010;2:144–8.
Zhou GLYL. Reduced BMP6 expression by DNA methylation contributes to EMT and drug resistance in breast cancer cells. Oncol Rep. 2014;2:581–8.
Settleman ASAJ. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;34:4741–51.
Sarkar FH, YLZW. Pancreatic cancer stem cells and EMT in drug resistance and metastasis. Minerva Chir. 2009;5:489–500.
Neel DS, Bivona TG. Secrets of drug resistance in NSCLC exposed by new molecular definition of EMT. Clin Cancer Res. 2013;19:3–5.
Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metast Rev. 2009;28:65–76.
Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, et al. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci. 2014;111:E384–93.
Baranwal S, Alahari SK. MiRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–90.
Weber M, Kim S, Patterson N, Rooney K, Searles CD. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306:H1192–203.
Dagan LN, Jiang X, Bhatt S, Cubedo E, Rajewsky K, Lossos IS. MiR-155 regulates HGAL expression and increases lymphoma cell motility. Blood. 2012;119:513–20.
Shi JS, Zhang J, Li J. Role of miR-155 in pathogenesis of diffuse large B cell lymphoma and its possible mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:869–72.
Zhang B, Zhang Y, Dagher MC, Shacter E. Rho GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res. 2005;65:6054–62.
Ohmine K, Nagai T, Tarumoto T, Miyoshi T, Muroi K, Mano H, et al. Analysis of gene expression profiles in an imatinib-resistant cell line, KCL22/SR. Stem Cells. 2003;21:315–21.
Doublier S, Riganti C, Voena C, Costamagna C, Aldieri E, Pescarmona G, et al. RhoA silencing reverts the resistance to doxorubicin in human colon cancer cells. Mol Cancer Res. 2008;6:1607–20.
Kobune M, Chiba H, Kato J, Kato K, Nakamura K, Kawano Y, et al. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol Cancer Ther. 2007;6:1774–84.
Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–46.
Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–44.
Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737.
Hommes DW, Peppelenbosch MP, van Deventer SJH. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–51.
Zhu J, Chen T, Yang L, Li Z, Wong MM, Zheng X, et al. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551.
Rahadiani N, Takakuwa T, Tresnasari K, Morii E, Aozasa K. Latent membrane protein-1 of Epstein-Barr virus induces the expression of B-cell integration cluster, a precursor form of microRNA-155, in B lymphoma cell lines. Biochem Biophys Res Commun. 2008;377:579–83.
Donnelly SM, Paplomata E, Peake BM, Sanabria E, Chen Z, Nahta R. P38 MAPK contributes to resistance and invasiveness of HER2- overexpressing breast cancer. Curr Med Chem. 2014;21:501–10.
Mei MXDZY. A new 2a,5a,10b,14b-tetraacetoxy-4(20),11-taxadiene (SIA) derivative overcomes paclitaxel resistance by inhibiting MAPK signaling and increasing paclitaxel accumulation in breast cancer cells. Plos One. 2014;8:e104317.
Donovan JC, Milic A, Slingerland JM. Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation and antiestrogen resistance in human breast cancer cells. J Biol Chem. 2001;276:40888–95.
Heckler MM, HTCC. ERK/MAPK regulates ERRc expression, transcriptional activity and receptor-mediated tamoxifen resistance in ER+ breast cancer. FEBS J. 2014; 22442–24341.
Normanno N, Campiglio M, Maiello MR, De Luca A, Mancino M, Gallo M, et al. Breast cancer cells with acquired resistance to the EGFR tyrosine kinase inhibitor Gefitinib show persistent activation of MAPK signaling. Breast Cancer Res Treat. 2008;112:25–33.
Nathan Corbett M, Martin Alda M. On telomeres long and short. J Psychiatry Neurosci. 2015;1:3–4.
Misteli T. The long reach of telomeres. Genes Dev. 2014;28:2445–6.
Dinami R, Ercolani C, Petti E, Piazza S, Ciani Y, Sestito R, et al. MiR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer Res. 2014;74:4145–56.
Cerone MA. Telomerase inhibition enhances the response to anticancer drug treatment in human breast cancer cells. Mol Cancer Ther. 2006;5:1669–75.
Ward RJ. Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol. 2005.
Lu L, Zhang C, Zhu G, Irwin M, Risch H, Menato G, et al. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13:R56.
Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1236–43.
Sochor M, Basova P, Pesta M, Dusilkova N, Bartos J, Burda P, et al. Oncogenic microRNAs: MiR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer. 2014;14:448.
Acknowledgments
This work has no fund. We thank Shan-Liang Zhong, M.D., and Wei-Xian Chen, PhD, for their discussion and help in revision.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, Dd., Lv, Mm., Chen, Wx. et al. Role of miR-155 in drug resistance of breast cancer. Tumor Biol. 36, 1395–1401 (2015). https://doi.org/10.1007/s13277-015-3263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3263-z