Abstract
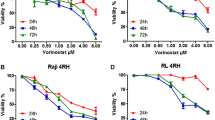
Suberoylanilide hydroxamic acid (SAHA; vorinostat), the second generation of histone deacetylase (HDAC) inhibitor, has been approved for the treatment of cutaneous manifestations of cutaneous T cell lymphoma (CTCL). It has also shown its anticancer activity over a large range of other hematological and solid malignancies, but few studies have been reported in B cell lymphoma. In this study, we aimed to investigate the antitumor activity of SAHA on murine B cell lymphoma cell line A20 cells. We treated A20 cells with different concentrations of SAHA. The effect of SAHA on the proliferation of A20 cells was studied by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) assay in vitro; the anti-proliferation activity in vivo was evaluated by proliferating cell nuclear antigen (PCNA) of xenograft tumor tissues through immunocytochemical staining. Apoptosis were detected by Hoechst 33258 staining and Annexin V/propidium iodide (PI) double-labeled cytometry in vitro. The effect of SAHA on cell cycle of A20 cells was studied by a propidium iodide method. Autophagic cell death induced by SAHA was confirmed by transmission electron microscopy (TEM). Angiogenesis marker (CD31) was measured by immunocytochemical staining to investigate the anti-angiogenic effect of SAHA. Western blot was used to detect the expression of signaling pathway factors (phospho-AKT, phospho-ERK, AKT, ERK, Nur77, HIF-1α, and VEGF). Our results showed that SAHA inhibited the proliferation of A20 cells in a time- and dose-dependent manner, induced cell apoptosis and G0/G1 phase arrest of cell cycle, promoted autophagic cell death, and suppressed tumor progress in NCI-A20 cells nude mice xenograft model in vivo. SAHA decreased the activation of AKT (phospho-AKT: p-AKT) and ERK1/2 (phospho-ERK: p-ERK) proteins and inhibited the expression of pro-angiogenic factors (VEGF and HIF-1α), downregulated its downstream signaling factor (Nur77), which might be contributed to the antitumor mechanisms of SAHA.







Similar content being viewed by others
References
Li Q, Burgess R, Zhang Z. All roads lead to chromatin: multiple pathways for histone deposition. Biochim Biophys Acta. 2012;1819:238–46.
Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–33.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705.
Nguyen HN, Kim JH, Jeong CY, Hong SW, Lee H. Inhibition of histone deacetylation alters arabidopsis root growth in response to auxin via pin1 degradation. Plant Cell Rep. 2013;32:1625–36.
Ververis K, Hiong A, Karagiannis TC, Licciardi PV. Histone deacetylase inhibitors (hdacis): multitargeted anticancer agents. Biologics. 2013;7:47–60.
Chen WL, Sheu JR, Hsiao CJ, Hsiao SH, Chung CL, Hsiao G. Histone deacetylase inhibitor impairs plasminogen activator inhibitor-1 expression via inhibiting tnf-alpha-activated mapk/ap-1 signaling cascade. Biomed Res Int. 2014;2014:231012.
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous t-cell lymphoma. Oncologist. 2007;12:1247–52.
Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–6.
Mazumder A, Vesole DH, Jagannath S. Vorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: a case series illustrating utility in clinical practice. Clin Lymphoma Myeloma Leuk. 2010;10:149–51.
Karelia N, Desai D, Hengst JA, Amin S, Rudrabhatla SV, Yun J. Selenium-containing analogs of SAHA induce cytotoxicity in lung cancer cells. Bioorg Med Chem Lett. 2010;20:6816–9.
Hurwitz JL, Stasik I, Kerr EM, Holohan C, Redmond KM, McLaughlin KM, et al. Vorinostat/saha-induced apoptosis in malignant mesothelioma is flip/caspase 8-dependent and hr23b-independent. Eur J Cancer. 2012;48:1096–107.
Siegel D, Hussein M, Belani C, Robert F, Galanis E, Richon VM, et al. Vorinostat in solid and hematologic malignancies. J Hematol Oncol. 2009;2:31.
Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107:14639–44.
Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–3.
Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors fk228 and cbha-induced apoptosis. Cell Death Differ. 2006;13:129–40.
Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the rb-e2f1 pathway for apoptosis induction through activation of proapoptotic protein bim. Proc Natl Acad Sci U S A. 2005;102:16090–5.
Liang D, Kong X, Sang N. Effects of histone deacetylase inhibitors on hif-1. Cell Cycle. 2006;5:2430–5.
Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–36.
Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:673–8.
Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6.
Lai JP, Sandhu DS, Shire AM, Roberts LR. The tumor suppressor function of human sulfatase 1 (sulf1) in carcinogenesis. J Gastrointest Cancer. 2008;39:149–58.
Ilieva H, Nagano I, Murakami T, Shiote M, Shoji M, Abe K. Sustained induction of survival p-akt and p-erk signals after transient hypoxia in mice spinal cord with g93a mutant human sod1 protein. J Neurol Sci. 2003;215:57–62.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27.
Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, et al. Erk1 and erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4:e8283.
Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via erk- and akt-dependent pathways. Biochim Biophys Acta. 1803;2010:1106–13.
Chen CS, Weng SC, Tseng PH, Lin HP. Histone acetylation-independent effect of histone deacetylase inhibitors on akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem. 2005;280:38879–87.
Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochimica Biophys Acta (BBA) - Mol Cell Res. 2009;1793:1516–23.
Chen N, Debnath J. Autophagy and tumorigenesis. FEBS Lett. 2010;584:1427–35.
Kobayashi S, Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2015;1852:252–61.
Lee M-S. Role of islet β cell autophagy in the pathogenesis of diabetes. Trends Endocrinol Metab. 2014;25:620–7.
Vidal RL, Matus S, Bargsted L, Hetz C. Targeting autophagy in neurodegenerative diseases. Trends Pharmacol Sci. 2014;35:583–91.
Ni B-B, Li B, Yang Y-H, Chen J-W, Chen K, Jiang S-D, et al. The effect of transforming growth factor β1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine. 2014;70:87–96.
Zhou X, Münger K. Expression of the human papillomavirus type 16 e7 oncoprotein induces an autophagy-related process and sensitizes normal human keratinocytes to cell death in response to growth factor deprivation. Virology. 2009;385:192–7.
Zhang Q, Zen K. Chapter 21—hypoxia-induced autophagy promotes tumor cell survival. In: Hayat MA, editor. Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging. Amsterdam: Academic; 2014. p. 305–17.
Liang N, Jia L, Liu Y, Liang B, Kong D, Yan M, et al. Atm pathway is essential for ionizing radiation-induced autophagy. Cell Signal. 2013;25:2530–9.
Gewirtz DA, Hilliker ML, Wilson EN. Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol. 2009;92:323–8.
Hashimoto D, Bläuer M, Sand J, Hirota M, Laukkarinen J. The role of autophagy in pancreatic cancer cells (panc-1) treated with anticancer drugs and inhibitors of autophagy. Pancreatology. 2013;13:e32–3.
Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2004;101:18030–5.
Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17.
Richter-Landsberg C, Leyk J. Inclusion body formation, macroautophagy, and the role of hdac6 in neurodegeneration. Acta Neuropathol. 2013;126:793–807.
Oh M, Choi I-K, Kwon HJ. Inhibition of histone deacetylase1 induces autophagy. Biochem Biophys Res Commun. 2008;369:1179–83.
Li J, Liu R, Lei Y, Wang K, Lau QC, Xie N, et al. Proteomic analysis revealed association of aberrant ros signaling with suberoylanilide hydroxamic acid-induced autophagy in jurkat t-leukemia cells. Autophagy. 2010;6:711–24.
Deepak AV, Salimath BP. Antiangiogenic and proapoptotic activity of a novel glycoprotein from U. indica is mediated by nf-kappab and caspase activated DNase in ascites tumor model. Biochimie. 2006;88:297–307.
Marme D. Tumor angiogenesis: the pivotal role of vascular endothelial growth factor. World J Urol. 1996;14:166–74.
Sumpio BE, Yun S, Cordova AC, Haga M, Zhang J, Koh Y, et al. Mapks (erk1/2, p38) and akt can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (cd31) in vascular endothelial cells. J Biol Chem. 2005;280:11185–91.
Khattab AZ, Ahmed MI, Fouad MA, Essa WA. Significance of p53 and cd31 in astrogliomas. Med Oncol. 2009;26:86–92.
Martinez-Gonzalez J, Badimon L. The nr4a subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–18.
Ismail H, Mofarrahi M, Echavarria R, Harel S, Verdin E, Lim HW, et al. Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77. Arterioscler Thromb Vasc Biol. 2012;32:1707–16.
Bras A, Albar JP, Leonardo E, de Buitrago GG, Martinez AC. Ceramide-induced cell death is independent of the fas/fas ligand pathway and is prevented by nur77 overexpression in a20 b cells. Cell Death Differ. 2000;7:262–71.
Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, et al. Orphan nuclear receptor tr3/nur77 regulates vegf-a-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–29.
Deutsch AJ, Rinner B, Wenzl K, Pichler M, Troppan K, Steinbauer E, et al. Nr4a1-mediated apoptosis suppresses lymphomagenesis and is associated with a favorable cancer-specific survival in patients with aggressive b-cell lymphomas. Blood. 2014;123:2367–77.
Acknowledgments
Thanks are expressed to the Laboratory of Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, and for the laboratory of Electron Microscopy of Tongji Medical College for offering relevant experimental facilities and technical support.
This work was supported by the Science Foundation of Hubei Health Department (No. JX6B08), Wujieping Medical Foundation (No.320.6750.13277), and National Nature Science Foundation of China (No. 81472707).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, B., Yu, D., Liu, J. et al. Antitumor activity of SAHA, a novel histone deacetylase inhibitor, against murine B cell lymphoma A20 cells in vitro and in vivo. Tumor Biol. 36, 5051–5061 (2015). https://doi.org/10.1007/s13277-015-3156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3156-1