Abstract
Sphingolipids are membrane and intracellular lipids that typically modulate cellular processes to cause cell death. Exogenous administration of sphingolipids may cause restriction of tumour growth and several alternative strategies are being used to control the cell growth. The microbes, their cellular component(s) or metabolites like DHA, EPA and also FTY720 have been employed as new therapeutic entities to regulate the disease condition. The therapeutic efficacy of lipids from Leishmania donovani in rheumatoid arthritis and also in sepsis condition associated with inflammatory diseases is well established. In this study, we explored the apoptotic effect of LSPL-1 (leishmanial sphingolipid-1) in Sarcoma 180 cells towards the regulation of tumour growth. The study using a panel of cancer cell lines revealed that LSPL-1 induces cell death in Sarcoma 180. The apoptotic changes were assessed by annexin exposure and DNA content analysis using flow cytometry. LSPL-1 appears to activate several pro- and anti-apoptotic molecules through reactive oxygen species (ROS) generation and also caspase activation, as determined by Western blot and ELISA analyses. Simultaneously, it may improve the survival rate of mice bearing tumour induced by Sarcoma 180 cells, with pathological changes. LSPL-1 may also suppress the cancer-associated inflammatory responses with the expression of matrix metalloproteinase having inhibitory role. It may regulate several angiogenic factors including VEGF, Ang-2 and CD34 in angiogenic events generated in Sarcoma 180 cell-induced tumour. These studies underline the significance of anti-neoplastic potential of LSPL-1 through apoptosis induction and abrogation of angiogenic responses in Sarcoma 180 cell-associated tumour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer appears to be a major cause of death worldwide, and the situation may take a turn for the worse due to the development of drug resistance. Thus, it requires better, alternative approaches for improvements in patient life [1, 2]. Most of chemotherapeutic agents in use originated from synthetic or natural source but are unable to restrict tumour cell growth [3]. To resolve this problem, the microbe-mediated therapeutic approach has been developed in recent years. Several microorganisms and their body parts, mainly enzymes, proteins and metabolites, are novel research tools to develop anti-tumour agents [4–6]. Interest in these therapeutic agents arose due to the discovery of several antibiotics like penicillin, amphotericin, etc. from microbial sources. Apoptosis induction is the major aim in developing anti-cancer agents to restrict tumour growth. For restricting tumour growth in therapeutic targets, the focus is presently on managing angiogenesis and inflammatory response via modulation of matrix metalloproteases [7]. Sphingolipids, specially their components like ceramides, may play various cellular functions including proliferation, differentiation, growth arrest and apoptosis. Applied exogenously, these sphingolipid components may inhibit cell growth in many cancer regulation cells or regulate the immunological system in cells [8–10].
Microbial cellular components like lipid, protein and their body components are emerging as a therapeutic target for many cancer types [11–13]. We very recently reported that the leishmanial lipid shows biological activity in in vitro and in vivo systems and also proves useful for rheumatoid arthritis patients through immune regulation [14, 15]. So, we have decided to find out if the sphingolipid isolated from Leishmania donovani can also fight against cancer by inducing apoptosis with simultaneous regulation of angiogenesis and inflammatory situation in in vivo systems.
Material and methods
Chemicals
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and p-nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate system (NBT-BCIP) were the products of SRL, India. Sarcoma 180 cell lines were obtained from ATCC, USA. Dulbecco’s modified Eagle medium (DMEM), foetal bovine serum (FBS), penicillin streptomycin neomycin (PSN) antibiotic, trypsin and ethylene diamine tetra acetic acid (EDTA) were obtained from Gibco BRL (Grand Island, NY, USA). Tissue culture plastic wares from NUNC (Roskidle, Denmark) and Bradford protein assay reagent from Fermentus, EU. DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) was procured from Invitrogen, California. The antibodies were from Santa Cruz Biotechnology Inc, San Diego, USA. The caspase-3 and caspase-9 activity assay kit was obtained from e-Bioscience Inc, while propidium iodide (PI) was purchased from Sigma-Aldrich, MO, USA.
Isolation of sphingolipids from Leishmania donovani promastigote cells
Leishmania donovani UR6 (MHO/IN/1978/UR6) was grown in Ray’s modified medium, and the total lipid was isolated by Bligh and Dyer extraction method. From the total lipid, a sphingolipid rich lipid fraction was separated by high-performance thin-layer chromatography (HPTLC) with the solvent system chloroform–methanol–water 90:10:1 (v/v/v). The presence of sphingolipids (leishmanial sphingolipid (LSPL)-1–3) was detected by spraying the HPTLC plate with benzidine reagent. The bands were marked and scraped to elute each sphingolipid through a small glass column [16–18].
Cell culture
Sarcoma 180 cells were grown in a humidified 5 % CO2 atmosphere at 37 °C in an incubator and cultured in DMEM medium supplemented with 10 % heat-inactivated calf serum, 100 IU/ml penicillin and 100 μg/ml streptomycin. Isolated LSPL was added to 10 % FBS containing media to achieve the desired final concentration, and the mixture was sonicated in a water bath sonicator for use in subsequent experiments.
MTT assay
MTT assay was done to evaluate cell viability. The cells were plated in 96-well plates and treated with or without different concentrations of LSPL-1–3 for 24 h. Four hours after the addition of MTT, the cells were lysed, formazan was solubilized with acidic isopropanol and the absorbance of the solution was measured at 595 nm using an ELISA reader [19].
Assessment of cell morphology
Cells grown in 6-well plates in DMEM supplemented with 10 % FBS for 24 h were treated with or without LSPL-1. Morphological changes were observed with an inverted phase contrast microscope (Model: OLYMPUS IX 70; Olympus Optical Co. Ltd., Sibuya-ku, Tokyo, Japan) and images were acquired [20].
Fluorescence microscopy
For the detection of nuclear damage or chromatin condensation, treated and untreated cells were stained with 10 μg/ml of DAPI in order to distinguish the live, apoptotic and necrotic ones and observed under fluorescence microscope; images were then acquired with excitation and emission wavelengths of 488 and 550 nm, respectively [21].
Quantification of apoptosis using annexin-V and analysis of cell cycle with PI
Apoptosis was assayed by using an annexin-V FITC apoptosis detection kit (Calbiochem, CA, USA). Briefly, cells were treated with or without LSPL-1, then washed and stained with PI and annexin-V FITC in accordance with the manufacturer’s instructions. The percentages of live, apoptotic and necrotic cells were determined by flow cytometric method.
Harvested cells were fixed overnight in 70 % ethanol at 4 °C, collected by centrifugation, resuspended in phosphate-buffered saline (PBS) containing 25 μg/ml RNase and 0.5 % Triton-×100 and incubated for 1 h at 37 °C. These were stained with 50 μg/ml PI for 15 min at 4 °C and analysed by a flow cytometer [22].
Detection of cell death by ELISA
Cell death was assayed using an ELISA-based cell detection kit from Roche Molecular Biochemicals (Mannheim, Germany).
Caspase-3, caspase-9 and cytochrome c assay
Caspases and cytosolic cytochrome c were assayed by commercially available colorimetric assay kits (BioVision Research Products, Mountain View, CA, USA) in accordance with the manufacturer’s instructions.
Western blot analysis
The cells and tissue lysates were separated by 10 % SDS–PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA) using standard electroblotting procedures. Membranes were then blocked and immunolabelled with primary antibodies at 4 °C overnight. Alkaline phosphatase-conjugated secondary antibodies and NBT-BCIP were used as chromogenic substrates [23].
Mitochondrial membrane potential measurement
To measure the mitochondrial membrane potential, treated or untreated cells were washed and incubated with Rhodamine 123 (5 μg ml−1). Emission at 535 nm was measured in a spectrofluorometer (LS50B; Perkin Elmer).
Animals
In vivo experiments were performed using BALB/c adult female mice, weighing 18–22 g and obtained from the Animal House of Indian Institute of Chemical Biology (Kolkata, India). The mice were housed at a laboratory temperature of 24 ± 1 °C with 40–80 % relative humidity and received food and water ad libitum; light–dark cycle was maintained. Care and maintenance of animals were done in adherence with the guidelines of the Institutional Animal Care and Use Committee.
Anti-tumour activity in Sarcoma 180 cell-transplanted mice
Sarcoma 180 cells were incubated at 37 °C with normal saline. Then, 0.3 ml of cell suspension was injected into the mouse abdominal cavity. After 7 days of incubation, the cell suspension was extracted from the abdominal cavity for transferring to the next generation. At the end, the cells were first centrifuged at 1200 rpm for 5 min to remove the clear supernatant, proper complete medium was added, counting was done under light microscope matching 1 × 106 ml−1 cell suspension and 0.2 ml of cell suspension injected into the right armpit of mouse. When tumours were visible (10 days), treatment of LSPL-1 (i.p.) was started with the selection of murine safe dose and continued up to 45 days. Animals were sacrificed on the last day and the survivability of mice was assayed and at the end of experiment; we sacrificed the animals followed by the collection of sample. After collection of tumour from each group, it was fixed and stained by H&E for the microvessel count for mitotic index assessment [20, 24].
Histocytochemical study of apoptosis in tumour tissue
Tumour apoptosis was assessed by in situ end labelling of DNA fragments (TdT-mediated dUTP-biotin nick end labelling, TUNEL) using a commercially available kit (Roche Applied Science). The fresh tumour tissue was fixed in 10 % formaldehyde for 24 h, dehydrated, paraffin embedded and cut into 5-μm-thick sections which were subsequently mounted on slides and rehydrated before staining with TUNEL for microscopic analysis. The mean number of apoptotic cells was counted in 10 randomly selected high-power fields [25].
Apoptosis index (AI) = (number of total cells) / (total number of cells counted) × 100
Immunohistochemical analysis of tumour tissue
For immunohistochemical study, 5-μm-thick tumour tissue sections were prepared to analyse VEGF, CD34, HIF-1α, Ang2, MMP-2 and MMP-9 by confocal laser-scanning microscope (Revolution XD Spinning Disk with an iXon 897 EMCCD camera) as stated earlier [23].
Results
Inhibition of Sarcoma 180 cell growth by leishmanial sphingolipid
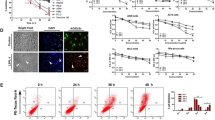
The bioactive sphingolipid(s) rich fraction was isolated from the total lipid of Leishmania donovani and subjected to fractionation with a silicic acid column. Spots visualized on HPTLC plate using sphingolipid specific reagent are shown in Fig. 1a. The cytotoxic effects of LSPL-1–3 on isolated Sarcoma 180 cells were determined by treating with 0–50 μg/ml concentrations for 0–48 h (Fig. 1b, c), which revealed dose- and time-dependent inhibition of cell growth; the optimum dose was found to be 50 μg/ml. So, 50 μg/ml was taken as the higher selected dose and 20 μg/ml as the lower one for subsequent experiments. No cytotoxicity was found in normal blood cells for 48 h at the concentration of 50 μg/ml (Fig. 1d).
Thin-layer chromatogram of leishmanial sphingolipids LSPL-1–3 visualized with benzidine spray (a). Growth inhibitory potential of LSPL (1–3) on Sarcoma 180 cells: concentration (0–50 μg/ml) (b) and time-dependent (0–48 h) (c) effects. The effect of LSPL-1 on normal cell (d). The data are reported as the mean ± SEM of triplicate experiments (*P > 0.05; **P > 0.01)
Morphological analysis of LSPL-1 in Sarcoma 180 cells
Analysis under phase contrast microscope revealed that after treatment with LSPL-1 at the dose of 50 μg/ml for 48 h, Sarcoma 180 cells underwent significant apoptotic changes like cell rounding, cell shrinkage and blebbing when compared with the respective control cells. Also, when visualized with DAPI and observed by fluorescence microscopy, cells treated with LSPL-1 showed condensed and fragmented nuclei unlike the untreated control showing bluish intact nuclei as in Fig. 2.
LSPL-1 induces apoptosis in Sarcoma 180 cells with annexin-V exposure and cell cycle arrest
It is well documented that the initial phase of apoptosis results in the release of annexin-V. Our study revealed that LSPL-1 at the concentrations 20 and 50 μg/ml, respectively, showed the highest number of apoptotic cells in the quadrants Q3 (0.71), Q2 (26.5) and Q3 (3.83) and Q2 (31.4) at the 48th hour as shown in Fig. 3a. At 50 μg/ml concentration, it also arrests the cell cycle in G0/G1 phase in 82 % cells and increases DNA fragmentation time dependently as determined by ELISA and shown in Figs. 3b and 3c. So, the study indicated that LSPL-1 induces apoptosis through cell cycle arrest in G0/G1 phase with induction of apoptosis.
Effect of LSPL-1 on annexin-V FITC binding (a) and cell cycle arrest (b) in two different concentrations for 48 h. Dose- and time-dependent DNA fragmentation (c) in Sarcoma 180 cells, measured as O.D. at 405 nm. The data are reported as the mean ± SEM of triplicate experiments (*P > 0.05; **P > 0.01)
LSPL-1 induces apoptosis in Sarcoma 180 cells through mitochondrial pathway
Apoptosis may follow extrinsic or intrinsic pathway. Treatment with 20 and 50 μg/ml of LSPL-1 for 48 h altered mitochondrial membrane potential as determined by changes in the ratio of PE-Texas Red and FITC-A. Flow cytometric analysis revealed 7.56 and 42.36 % of FITC-positive cells in A quadrant with respective increment of reactive oxygen species (ROS) generation up to 85 % in LSPL-1 (50 μg/ml)-treated cells as found in Fig. 4a, b. Release of cytochrome C occurred in time-dependent manner as shown in Fig. 4e with the activation of cystein protease family, mainly caspases 3 and 9, as shown in Fig. 4c, d. These studies indicated that LSPL-1 promoted apoptosis following the intrinsic pathway. On the other hand, immunoblot analysis of Sarcoma 180 cells at 48th hour of LSPL-1 (50 μg/ml) treatment showed upregulation of several pro-apoptotic proteins and downregulation of the anti-apoptotic factors with consequent cleavage of PARP molecule during the apoptosis process as found in Fig. 4f.
Flow cytometric evaluation of mitochondrial membrane potential using JC-1 (a) and assessment of intracellular ROS generation by H2DCFDA (b) in Sarcoma 180 cells with respective concentrations for 48 h. Assessment by ELISA of activation of caspases 3 and 9 (c, d) with cytochrome c (e) release by LSPL-1 in time dependent manner. Assessment of pro- and anti-apoptotic molecules by treatment of LSPL-1 was performed by western blot (f). The data are reported as the mean ± SEM of triplicate experiments (*P > 0.05; **P > 0.01)
Effect of LSPL-1 in tumour growth in murine system
The effect of LSPL-1 on growth regulation was observed in Sarcoma 180 cell-induced solid tumour. In this study, non-toxic doses of 25 and 50 mg/kg were selected for in vivo experiments. LSPL-1 at 50 mg/kg dose improved the survival rate of tumour bearing mice and reduced tumour growth dose dependently as evident from Fig. 5b, c. Figure 5d–f describes the decrease in mitotic index and microvascular density number on 55th day with treatment of LSPL-1 (50 mg/kg), determined by histological study. LSPL-1 (50 mg/kg) also induced apoptosis in tumour tissue as shown in Fig. 5g, which was confirmed by TUNEL assay, and the generation of apoptosis index is shown in Table 1.
Effect of different concentrations of LSPL-1, i.e. LSPL-1-D1 (25 mg/kg) and LSPL-1-D2 (50 mg/kg) to regulate tumour growth in Sarcoma 180 cell-treated mice. Photograph of tumour-bearing mice (a) grouped accordingly (I = control, II = Sarcoma 180 cells, III = Sarcoma 180 cells + LSPL-1-D1, IV = Sarcoma 180 cells + LSPL-1-D2). Percent survival (b) and tumour weight (c) of mice treated with Sarcoma 180 cells following administration of LSPL-1. H&E stain of tumour section (d). Arrows indicate the microvascular density with mitotic index of each group to identify the mitotic index (e) and tumour vasculature (f) by ten representative areas of each tumour section with ×10 magnification, (scale bars, 20 m). Apoptosis index from tumour tissue assayed by immunohistochemical method, indicated by arrows (g). The data are reported as the mean ± SEM of triplicate experiments (*P > 0.05; **P > 0.01)
Effect of LSPL-1 treatment on regulation of tumour angiogenesis with modulation of inflammation
Angiogenesis is the major contributor to tumour growth promotion. The expression levels of angiogenic factors like VEGF, CD34 and Ang-2 and the enhancement of transcription factor HIF-1α were significantly subdued in the tumour tissue with the treatment of LSPL-1 (50 mg/kg) when compared with tumour-bearing mice receiving no drug. Moreover, MMPs play a regulatory role in angiogenesis process from breakdown of basement membrane to high tumour growth promoting condition, especially through involvement of MMP-2 and MMP-9. Results of immunohistochemical analysis depicted in Fig. 6a–c show that the expression level of these MMPs was reduced dose dependently upon treatment of LSPL-1. This study also revealed that the levels of TNF-α and IL-6, which were subdued during tumour promotion, perhaps decrease the inflammatory responses upon treatment of LSPL-1 (50 mg/kg) as evidenced by immunoblot analysis (Fig. 6d).
Effect of LSPL-1-D1 (25 mg/kg) and LSPL-1-D2 (50 mg/kg) in regulation of angiogenesis in tumour-bearing mice. Immunofluorescence was used to localize VEGF and CD34 (a), HIF-1α and Ang-2 (b) in tissue section, and cell adhesion molecules ICAM-1 and VCAM-1 in tumour tissue (c). Immunoblot analysis of tumour tissue associated cytokines (d). The data are reported as the mean ± SEM of triplicate experiments (*P > 0.05; **P > 0.01)
Discussion
Alternative therapeutic strategies need to be developed to restrict cancer cell progression. The recent studies primarily focus on finding out agents of plant origin or of synthetic and semi-synthetic origin. But microbe-mediated therapy for restriction of tumour growth is a novel and current approach [26, 27]. Bacterial components such as endotoxin, protein or cellular components may participate in tumour cell destruction and also in vaccine development for cancer [28]. Importantly, bacteria or its modified version may be used as carrier for delivering the therapies in cancer treatment [29]. The latest example in microbe-mediated therapy is FTY720 which is used in multiple sclerosis; recently, it has been established as an anti-proliferative agent in regulating the growth of several cancer cells via the involvement of apoptotic response [30, 31]. Another example is the sophorolipid produced from a yeast strain that has shown apoptosis-inducing properties in human liver cancer cells and beneficial effect in bacterial sepsis also [32, 33]. We are also developing a new entity, i.e. leishmanial lipid, which has already been reported to have apoptosis-inducing effect in synovial cells isolated from rheumatoid arthritis patients [15]. Recently, we have reported the protective role of this lipid against Escherichia coli-induced bacterial sepsis [14]. Thus, the previous findings encouraged us to find out new biological efficacy of this leishmanial lipid in regulating cancer growth. According to our knowledge, this is the first report to show that LSPL-1 mediates apoptosis in Sarcoma 180 cells with inhibition of tumour growth in regulating angiogenesis. Here, we have described the in vitro study which indicated the inhibition of cancer cell proliferation along with apoptosis induction in time- and dose-dependent manner. Preliminary observation from morphological evaluation of Sarcoma 180 cells treated with LSPL-1 demonstrates the sequential phenomena of apoptosis like cellular death and phosphatidyl serine exposure from cell surface [20]. Cell cycle check point arrest is another incidental phenomenon during apoptosis that occurs as an effect of chemotherapeutic agents in target cells [22]. In our study, cell cycle analysis also reflected the cell cycle arrest at G0/G1 phase upon treatment of LSPL-1. This indicated increment in DNA content in respective phase in due time.
ROS are major contributors of apoptosis upon external stimuli and mitochondria are the major source for internal cellular ROS generation via activation of several signalling cascades. Alteration of mitochondrial homeostasis releases cytochrome c which is a major factor of apoptosis-induced cell death. Our study revealed that LSPL-1-induced apoptosis may alter mitochondrial membrane potential with generation of ROS by increasing the level of cytochrome C.
The biochemical effects of caspase signalling are considered as the contributors to mitochondrial apoptosis. We found that LSPL-1 eventually activates caspases 9 and 3 during apoptosis. It is already reported that bacterial ceramide may induce apoptosis via activation of mitochondrial pathway. In vivo study also has focused on Sarcoma 180 cell-induced tumour induction in BALB/c mice. Upon treatment with LSPL-1, the survival rate of mice improved with reduction of tumour weight and volume; several histopathological changes were noted in tumour tissue including those in mitotic index and microvascular density. Moreover, several cytokines, chemokines and transcription factors and specially MMPs regulate the tumour micro-environment in in vivo systems. Herein, we focused on tumour angiogenesis to evaluate the effect of LSPL-1 in Sarcoma 180-induced tumour. We found that the expression levels of biomarkers VEGF and CD34 were reduced in tumour-bearing mice after LSPL-1 treatment.
During angiogenesis, the induction of cell proliferation, increased expression of cell adhesion molecules, increased secretion of MMPs and increased migration and invasion gear up the progression of metastasis. Importantly, MMPs are degraded in the basement membrane to allow endothelial cells to detach and migrate to new tissues by releasing angiogenic factors which modulate the signalling system of cell adhesion molecules. During cancer promotion, MMPs are involved in initiating inflammation through progression of cancer with involvement of cytokine series and transcriptional factors. LSPL-1 also reduced the elevated levels of cytokines and chemokines which are mainly involved in cell proliferation to tumourigenesis through tumour-associated inflammatory responses.
Interestingly, our data convincingly showed that LSPL-1 initiated the induction of apoptosis in Sarcoma 180 cells via alteration of mitochondrial homeostasis with activation of caspases in vitro. Also the in vivo study indicated reduction of tumour volume and restriction of angiogensis with regulation of MMP and cytokine–chemokine circuit.
In conclusion, we suggest that leishmanial sphingolipid component is a potential entity for restriction of cancer cell growth with involvement of several apoptotic molecules and tumour growth factors without any adverse effect on the system. Further investigation is needed to explore its other tumour-targeting properties.
Approximately 100 cells were counted per field, five fields were examined per slide and five slides were examined per group.
References
Dyzmann-Sroka A, Malicki J: Cancer incidence and mortality in the greater Poland region-analysis of the year 2010 and future trends. Rep Pract Oncol Radiother;19:296–300
Prasad V, Goldstein JA: US news and world report cancer hospital rankings: do they reflect measures of research productivity? PLoS One;9:e107803
Raguz S, Yague E. Resistance to chemotherapy: new treatments and novel insights into an old problem. Br J Cancer. 2008;99:387–91.
Chakrabarty AM. Microorganisms and cancer: quest for a therapy. J Bacteriol. 2003;185:2683–6.
Wei MQ, Mengesha A, Good D, Anne J. Bacterial targeted tumour therapy—dawn of a new era. Cancer Lett. 2008;259:16–27.
Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T, et al. Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999;59:482–6.
Bachmeier BE, Iancu CM, Jochum M, Nerlich AG. Matrix metalloproteinases in cancer: comparison of known and novel aspects of their inhibition as a therapeutic approach. Expert Rev Anticancer Ther. 2005;5:149–63.
Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–28.
Baumruker T, Prieschl EE. Sphingolipids and the regulation of the immune response. Semin Immunol. 2002;14:57–63.
Maceyka M, Spiegel S: Sphingolipid metabolites in inflammatory disease. Nature;510:58–67
Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–21.
Heimli H, Hollung K, Drevon CA. Eicosapentaenoic acid-induced apoptosis depends on acyl CoA-synthetase. Lipids. 2003;38:263–8.
Azuma H, Horie S, Muto S, Otsuki Y, Matsumoto K, Morimoto J, et al. Selective cancer cell apoptosis induced by fty720; evidence for a bcl-dependent pathway and impairment in erk activity. Anticancer Res. 2003;23:3183–93.
Das S, Chatterjee N, Bose D, Banerjee S, Pal P, Jha T, Das Saha K: Lipid isolated from a Leishmania donovani strain reduces Escherichia coli induced sepsis in mice through inhibition of inflammatory responses. Mediat Inflamm;2014:409694
Majumdar KN, Banerjee A, Ratha J, Mandal M, Sarkar RN, Saha KD. Leishmanial lipid suppresses tumor necrosis factor alpha, interleukin-1beta, and nitric oxide production by adherent synovial fluid mononuclear cells in rheumatoid arthritis patients and induces apoptosis through the mitochondrial-mediated pathway. Arthritis Rheum. 2008;58:696–706.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Dasgupta S, Hogan EL. Chromatographic resolution and quantitative assay of cns tissue sphingoids and sphingolipids. J Lipid Res. 2001;42:301–8.
Bischel MD, Austin JH. A modified benzidine method for the chromatographic detection of sphingolipids and acid polysaccharides. Biochim Biophys Acta. 1963;70:598–600.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Das S, Chatterjee N, Bose D, Dey SK, Munda RN, Nandy A, Bera S, Biswas SK, Das Saha K: Anticancer potential of 3-(arylideneamino)-2-phenylquinazoline-4(3H)-one derivatives. Cell Physiol Biochem, 29:251–260
Pramanik M, Chatterjee N, Das S, Saha KD, Bhaumik A: Anthracene-bisphosphonate based novel fluorescent organic nanoparticles explored as apoptosis inducers of cancer cells. Chem Commun (Camb);49:9461–9463.
Mandal SCN, Das S, Saha KD, Chaudhuri K. Magnetic core-shell nanoprobe for sensitive killing of cancer cells via induction with a strong external magnetic field. RSC Adv. 2014;39:20077–85.
Chatterjee N, Das S, Bose D, Banerjee S, Jha T, Saha KD: Leishmanial lipid suppresses the bacterial endotoxin-induced inflammatory response with attenuation of tissue injury in sepsis. J Leukoc Biol;96:325–336.
Ji DB, Ye J, Jiang YM, Qian BW: Anti-tumor effect of liqi, a traditional chinese medicine prescription, in tumor bearing mice. BMC Complement Altern Med 2009;9:20
Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10.
Minton NP. Clostridia in cancer therapy. Nat Rev Microbiol. 2003;1:237–42.
Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98:15155–60.
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–70.
Saltzman DA, Heise CP, Hasz DE, Zebede M, Kelly SM, Curtiss 3rd R, et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases mc-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm. 1996;11:145–53.
Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. Fty720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–84.
Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ: Fty720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy;7:707–715
Fu SL, Wallner SR, Bowne WB, Hagler MD, Zenilman ME, Gross R, et al. Sophorolipids and their derivatives are lethal against human pancreatic cancer cells. J Surg Res. 2008;148:77–82.
Hardin R, Pierre J, Schulze R, Mueller CM, Fu SL, Wallner SR, et al. Sophorolipids improve sepsis survival: effects of dosing and derivatives. J Surg Res. 2007;142:314–9.
Acknowledgments
The Council of Scientific and Industrial Research, India financially supported this work. The authors convey their sincerest thanks to the Director, Dr. Chitra Mandal of CSIR-Indian Institute of Chemical Biology for providing us the necessary support for this work. We extend our thanks to Dr. Suvendra Nath Bhattacharyya (Indian Institute of Chemical Biology) and Mr. Diptadeep Sarkar for their help in confocal laser-scanning microscopy and Dr S. N. Kabir and Anushila Gangopadhyay for flow cytometry. We are also indebted to Dr. Basudeb Achari and Dr. J. Rajan Vedasiromoni of our institute for critically reviewing the manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, S., Chatterjee, N., Bose, D. et al. Leishmanial sphingolipid induces apoptosis in Sarcoma 180 cancer cells through regulation of tumour growth via angiogenic switchover. Tumor Biol. 36, 3109–3118 (2015). https://doi.org/10.1007/s13277-014-2947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2947-0