Abstract
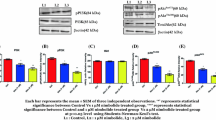
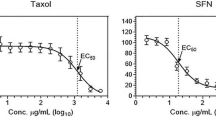
The purposes of this study are to investigate the antitumor activities of NSK-01105, a novel sorafenib derivative, in in vitro and in vivo models, and explore the potential mechanisms. The effects of NSK-01105 on proliferation and apoptosis of prostate cancer cells were established by cytotoxicity assays, apoptosis analysis, flow cytometry analysis, and Western blot analysis. Two xenograft tumor models were used to verify the therapeutic effect of NSK-01105 in vivo. NSK-01105 exhibited broad-spectrum antitumor activity, particularly in prostate cancer cells. Characterization of apoptosis morphology was observed, and the percentage of apoptosis-positive cells significantly increased after NSK-01105 treatment for 24 h. Furthermore, a significant increase of the “sub-G1” population in LNCaP and PC-3 cells after NSK-01105 treatment was determined by cell cycle analysis. Tumor growth was significantly suppressed by once daily oral 30 mg/kg dose of NSK-01105 with the inhibition rates of 63.82 % in LNCaP models and 64.29 % in PC-3 models, respectively. The activation of Raf-1 kinase and epidermal growth factor receptor was downregulated by NSK-01105 at 10 μmol/L. Consequently, the dual inhibitions of Raf/MEK/ERK and PI3K/Akt/mTOR signal pathways were observed by Western blot analysis. Collectively, our results suggest a role of NSK-01105 in treatment for human prostate tumors by inhibiting cell proliferation and inducing apoptosis. NSK-01105 appears to be a promising orally active anticancer drug and deserves further investigation.






Similar content being viewed by others
References
Wallace TJ, Torre T, Grob M, Yu J, Avital I, Brucher B, et al. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer. 2014;5:3–24.
Vishnu P, Tan WW. Update on options for treatment of metastatic castration-resistant prostate cancer. Oncol Targets Ther. 2010;3:39–51.
Oh SJ, Erb HH, Hobisch A, Santer FR, Culig Z. Sorafenib decreases proliferation and induces apoptosis of prostate cancer cells by inhibition of the androgen receptor and Akt signaling pathways. Endocrinol Relat Cancer. 2012;19:305–19.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–34.
Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer. 2007;97:1480–5.
Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann Oncol. 2008;19:746–51.
Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–14.
Peraldo-Neia C, Migliardi G, Mello-Grand M, Montemurro F, Segir R, Pignochino Y, et al. Epidermal growth factor receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer. 2011;11:31.
Gravis G, Bladou F, Salem N, Goncalves A, Esterni B, Walz J, et al. Results from a monocentric phase II trial of erlotinib in patients with metastatic prostate cancer. Ann Oncol. 2008;19:1624–8.
Abouzid K, Shouman S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg Med Chem. 2008;16:7543–51.
Wang S, Liu Q, Zhang Y, Liu K, Yu P, Luan J, et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;8:81.
Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology. 2009;59:219–29.
Jiang N, Zhai X, Li T, Liu D, Zhang T, Wang B, et al. Design, synthesis and antiproliferative activity of novel 2-substituted-4-amino-6-halogenquinolines. Molecules. 2012;17:5870–81.
Ahn YT, Shin IJ, Kim JM, Kim YS, Lee C, Ju SA, et al. Counteracting the activation of pAkt by inhibition of MEK/Erk inhibition reduces actin disruption-mediated apoptosis in PTEN-null PC3M prostate cancer cell lines. Oncol Lett. 2013;6:1383–9.
Robertson BW, Bonsal L, Chellaiah MA. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol Cancer. 2010;9:260.
Fukuyo Y, Hunt CR, Horikoshi N. Geldanamycin and its anti-cancer activities. Cancer Lett. 2010;290:24–35.
Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003;3:371–7.
Gioeli D, Mandell JW, Petroni GR, Frierson Jr HF, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–84.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84.
Jull BA, Plummer 3rd HK, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–17.
Sabbah DA, Brattain MG, Zhong H. Dual inhibitors of PI3K/mTOR or mTOR-selective inhibitors: which way shall we go? Curr Med Chem. 2011;18:5528–44.
Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57.
Dan S, Okamura M, Seki M, Yamazaki K, Sugita H, Okui M, et al. Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res. 2010;70:4982–94.
Fu QH, Zhang Q, Bai XL, Hu QD, Su W, Chen YW, et al. Sorafenib enhances effects of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014.
Small EJ, Fontana J, Tannir N, DiPaola RS, Wilding G, Rubin M, et al. A phase II trial of gefitinib in patients with non-metastatic hormone-refractory prostate cancer. BJU Int. 2007;100:765–9.
Joensuu G, Joensuu T, Nokisalmi P, Reddy C, Isola J, Ruutu M, et al. A phase I/II trial of gefitinib given concurrently with radiotherapy in patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:42–9.
Vuky J, Porter C, Isacson C, Vaughan M, Kozlowski P, Picozzi V, et al. Phase II trial of neoadjuvant docetaxel and gefitinib followed by radical prostatectomy in patients with high-risk, locally advanced prostate cancer. Cancer. 2009;115:784–91.
Zhong H, Bowen JP. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr Top Med Chem. 2011;11:1571–90.
Aziz SA, Jilaveanu LB, Zito C, Camp RL, Rimm DL, Conrad P, et al. Vertical targeting of the phosphatidylinositol-3 kinase pathway as a strategy for treating melanoma. Clin Cancer Res. 2010;16:6029–39.
Sacco A, Roccaro A, Ghobrial IM. Role of dual PI3/Akt and mTOR inhibition in Waldenstrom’s macroglobulinemia. Oncotarget. 2010;1:578–82.
Wang XQ, Fan JM, Liu YO, Zhao B, Jia ZR, Zhang Q. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int J Pharm. 2011;419:339–46.
Acknowledgments
This research was supported by the National Basic Research Program of China (No. 2012CB724003) and National Natural Science Foundation of China (No. 81202038).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, P., Ye, L., Wang, H. et al. NSK-01105 inhibits proliferation and induces apoptosis of prostate cancer cells by blocking the Raf/MEK/ERK and PI3K/Akt/mTOR signal pathways. Tumor Biol. 36, 2143–2153 (2015). https://doi.org/10.1007/s13277-014-2824-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2824-x