Abstract
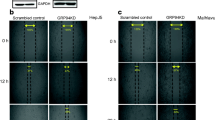
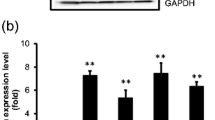
Glucose-regulated protein 78 (GRP78) is a key chaperone and stress response protein. Previous studies have demonstrated that high GRP78 expression may be correlated with cancer progression and therapeutic response. However, the role of GRP78 in the metastasis of colon cancer is unclear. In this study, we used small interfering RNA (siRNA) to knock down GRP78 expression in colon cancer cells (HT-29 and DLD-1 cells). In wound-healing migration assays, we found that GRP78-knockdown (GRP78KD) cells showed better wound-healing ability than control cells. We also found that GRP78KD cells displayed a better migratory ability than control cells in migration and invasion assays. As we further dissected the underlying molecular mechanism, we found that silencing GRP78 may cause an increase in vimentin expression and a decrease in the E-cadherin level, which was correlated with the increase in migratory ability. In addition, we found that GRP78KD may activate the NRF-2/HO-1 pathway, and this activation was also correlated with the increase in cell invasiveness. Furthermore, we examined GRP78 expression in a tissue array and found that the GRP78 expression in metastatic adenocarcinoma in lymph nodes tended to be weaker than that in primary colonic adenocarcinoma. In conclusion, a low level of GRP78 may cause an increase in metastasis ability in colon cancer cells by altering E-cadherin and vimentin expression and activating the NRF-2/HO-1 signaling pathway. Our study demonstrates that low expression of GRP78 may correlate with a high risk of metastasis in colon cancer.







Similar content being viewed by others
References
Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300.
Haas IG. Bip (grp78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia. 1994;50:1012–20.
Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–51.
Pfaffenbach KT, Lee AS. The critical role of grp78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–6.
Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54.
Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–40.
Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, et al. Overexpression of endoplasmic reticulum molecular chaperone grp94 and grp78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–51.
Langer R, Feith M, Siewert JR, Wester HJ, Hoefler H. Expression and clinical significance of Glucose Regulated Proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer. 2008;8:70.
Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–9.
Xing X, Lai M, Wang Y, Xu E, Huang Q. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–15.
Bifulco G, Miele C, Di Jeso B, Beguinot F, Nappi C, Di Carlo C, et al. Endoplasmic reticulum stress is activated in endometrial adenocarcinoma. Gynecol Oncol. 2012;125:220–5.
Song MS, Park YK, Lee JH, Park K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase c-epsilon/erk/ap-1 signaling cascade. Cancer Res. 2001;61:8322–30.
Park HR, Ryoo IJ, Choo SJ, Hwang JH, Kim JY, Cha MR, et al. Glucose-deprived HT-29 human colon carcinoma cells are sensitive to verrucosidin as a GRP78 down-regulator. Toxicology. 2007;229:253–61.
Chiou JF, Tai CJ, Huang MT, Wei PL, Wang YH, An J, et al. Glucose-regulated protein 78 is a novel contributor to acquisition of resistance to sorafenib in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:603–12.
Lee AS. Grp78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9.
Chang YJ, Huang YP, Li ZL, Chen CH. GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and Akt pathway after epirubicin treatment in colon cancer DLD-1 cells. PLoS ONE. 2012;7:e35123.
Kuo LJ, Hung CS, Chen WY, Chang YJ, Wei PL. Glucose-regulated protein 78 silencing down-regulates vascular endothelial growth factor/vascular endothelial growth factor receptor 2 pathway to suppress human colon cancer tumor growth. J Surg Res 2013
Takahashi H, Wang JP, Zheng HC, Masuda S, Takano Y. Overexpression of GRP78 and GRP94 is involved in colorectal carcinogenesis. Histol Histopathol. 2011;26:663–71.
Hardy B, Raiter A, Yakimov M, Vilkin A, Niv Y. Colon cancer cells expressing cell surface GRP78 as a marker for reduced tumorigenicity. Cell Oncol (Dordr). 2012;35:345–54.
Li Z, Zhang L, Zhao Y, Li H, Xiao H, Fu R, et al. Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int J Biochem Cell Biol. 2013;45:987–94.
Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16.
Hirsch FR, Varella-Garcia M, Bunn Jr PA, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–807.
Chang YJ, Chiu CC, Wu CH, An J, Wu CC, Liu TZ, et al. Glucose-regulated protein 78 (GRP78) silencing enhances cell migration but does not influence cell proliferation in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1703–9.
Wei PL, Kuo LJ, Huang MT, Ting WC, Ho YS, Wang W, et al. Nicotine enhances colon cancer cell migration by induction of fibronectin. Ann Surg Oncol 2011
Lien YC, Wang W, Kuo LJ, Liu JJ, Wei PL, Ho YS, et al. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann Surg Oncol 2011
Wei PL, Kuo LJ, Wang W, Lin FY, Liu HH, How T, et al. Silencing of glucose-regulated protein 78 (GRP78) enhances cell migration through the upregulation of vimentin in hepatocellular carcinoma cells. Ann Surg Oncol. 2012;19 Suppl 3:S572–9.
Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X. The role of Nrf2 in migration and invasion of human glioma cell U251. World Neurosurg. 2013;80:363–70.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Hwang JH, Kim JY, Cha MR, Ryoo IJ, Choo SJ, Cho SM, et al. Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down-regulator. J Cell Physiol. 2008;215:243–50.
Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18.
Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48.
Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for e-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3.
Wei PL, Chang YJ, Ho YS, Lee CH, Yang YY, An J, et al. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann Surg. 2009;249:978–85.
Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2012
Lu MH, Huang CC, Pan MR, Chen HH, Hung WC. Prospero homeobox 1 promotes epithelial–mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin Cancer Res. 2012;18:6416–25.
Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, et al. Introducing intermediate filaments: from discovery to disease. J Clin Invest. 2009;119:1763–71.
Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111(Pt 13):1897–907.
Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977–84.
Ivaska J. Vimentin: Central hub in EMT induction? Small GTPases. 2011;2:51–3.
Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol stimulates activation of snail, epidermal growth factor receptor signaling, and biomarkers of epithelial–mesenchymal transition in colon and breast cancer cells. Alcohol Clin Exp Res. 2010;34:19–31.
Zhou J, Yang J, Li K, Mo P, Feng B, Wang X, et al. RhoE is associated with relapse and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2013;20:175–82.
Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–18.
Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–35.
Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–34.
Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney Jr JW, et al. Induction of epithelial–mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–16.
Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. Journal of the American Society of Nephrology : JASN. 2001;12:47–53.
Li WP, Tsiokas L, Sansom SC, Ma R. Epidermal growth factor activates store-operated Ca2+ channels through an inositol 1,4,5-trisphosphate-independent pathway in human glomerular mesangial cells. J Biol Chem. 2004;279:4570–7.
Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–30.
Yang IH, Tsai YT, Chiu SJ, Liu LT, Lee HH, Hou MF, et al. Involvement of STIM1 and Orai1 in EGF-mediated cell growth in retinal pigment epithelial cells. J Biomed Sci. 2013;20:41.
Lai W, Liu L, Zeng Y, Wu H, Xu H, Chen S, et al. KCNN4 channels participate in the EMT induced by PRL-3 in colorectal cancer. Med Oncol. 2013;30:566.
Maines MD. The heme oxygenase system: past, present, and future. Antioxid Redox Signal. 2004;6:797–801.
Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, et al. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol. 2007;42:852–8.
Kang KA, Maeng YH, Zhang R, Yang YR, Piao MJ, Kim KC, et al. Involvement of heme oxygenase-1 in Korean colon cancer. Tumour Biol. 2012;33:1031–8.
Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116.
Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of nrf2. Pharmacol Rev. 2012;64:1055–81.
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420.
Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology 2008;135:1358–68, 1368 e1351-1354.
Akhdar H, Loyer P, Rauch C, Corlu A, Guillouzo A, Morel F. Involvement of Nrf2 activation in resistance to 5-fluorouracil in human colon cancer HT-29 cells. Eur J Cancer. 2009;45:2219–27.
Hu T, Yao Y, Yu S, Guo H, Han L, Wang W, et al. Clinicopathologic significance of CXCR4 and Nrf2 in colorectal cancer. J Biomed Res. 2013;27:283–90.
Acknowledgments
This study was supported by grants from the National Science Council (NSC101-2314-B-038-029-MY3 and NSC101-2314-B-038-016-MY3) and MOHW103-TDU-B-212-113001.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, YJ., Chen, WY., Huang, CY. et al. Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumor Biol. 36, 1859–1869 (2015). https://doi.org/10.1007/s13277-014-2788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2788-x