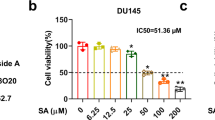
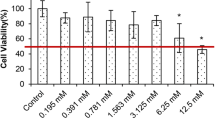
Abstract
Even though the role of lycopene from tomato (trans form) in controlling prostate cancer was reported, lycopene (cis and trans 60:40) isolated from green algae Chlorella marina was not reported so far. The present study aimed to assess the anti-proliferative and apoptotic effect of lycopene from a new source and to compare the activity with available trans lycopene by using androgen-independent human prostate cancer cell lines. Exposure of PC-3 and DU-145 cell lines to algal lycopene (AL) at a dose of 20 and 50 μM significantly inhibited the growth and colony formation, and the percentage of inhibition was higher than tomatal lycopene (TL)-treated groups. The stability of AL in cell culture medium was high, when compared to TL under standard cell culture conditions. The level of lycopene was not detected in PC-3 cell lines cultured in medium lacking lycopene. Staining cells with acridine orange and ethidium bromide, the PC-3 control cells showed largely non-fragmented intact nucleoid. Stronger apoptosis signal was induced with higher concentrations (50 μM) of algal lycopene. Increased DNA damage was observed in AL- and TL-treated cells which appear as comet during single-cell gel electrophoresis. Flow cytometry results revealed that AL caused PC-3 cells to accumulate in the G0/G1 phase and to undergo apoptosis. The effect was higher in AL groups than TL-treated groups. Algal lycopene showed very significant anti-proliferative and apoptotic effect in human prostate cancer cell lines. Therefore, algal lycopene from C.marina would be recommended for the treatment of prostate cancer.







Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300.
Bubendorf L, Schopfer A, Wagner U. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83.
Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81.
Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467–71.
Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51.
Lu QY, Hung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, et al. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomark Prev. 2001;10:749–56.
Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30.
Gerster H. The potential role of lycopene for human health. J Am Coll Nutr. 1997;16:109–26.
Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9.
Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med. 2002;227:881–5.
Bowen P, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, et al. Tomato sauce supplementation and prostate cancer: lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp Biol Med. 2002;227:886–93.
Kristal AR, Cohen JH. Invited commentary: tomatoes, lycopene, and prostate cancer. How strong is the evidence? Am J Epidemiol. 2000;151:124–30.
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr. 2001;131:3303–6.
Giovannucci E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp Biol Med. 2002;227:852–9.
Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–8.
Di Masico P, Kaiser S, Sies H. Lycopene as the most efficient carotenoids singlet oxygen quencher. Biochem Soc Trans. 1989;274:532–8.
Shi J, Le Mague M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40:1–42.
Aboul Enein AM, El-Baz FK, EI-Baroty GS, Youssef AM, Abd EI-Baky HH. Antioxidant activity of algal extracts on lipid peroxidation. J Med Sci. 2003;3:87–98.
Renju GL, Muraleedhara Kurup G, Saritha Kumari CH. Anti-inflammatory activity of lycopene from Chlorella marina on type II collagen induced arthritis in Sprague Dawley rats. Immunopharmacol Immunotoxicol. 2013;35(2):282–91.
Walne PR. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilis. Fish Investig. 1970;6:1–62.
Hk L. Chlorophylls and carotenoids: pigments of photosynthetic biomembrane. Methods Enzymol. 1987;147:350–82.
Fish WW, Veazie P, Collins JK. Extraction of lycopene from tomato paste. J Food Compos Anal. 2002;15:309–17.
Shaish A, Ben Amotz, Avron M. Biosynthesis of β carotene in Dunalialla. Methods Enzymol. 1992;213:439–44.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Creepy EE, Chiavappa P, Baudrimont I, Borracci P, Moukha, Carratu MR. Synergistic effects of fumonisin B1 and Aflatoxin B1: are invitro cytotoxicity data predictive of invivo acute toxicity? Toxicology. 2004;201:115–23.
Robbins DH, Itzkowitz SH. The molecular and genetic basis of colon cancer. Med Clin N Am. 2002;86:1467–95.
Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSHProtoc. 2006;3.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91.
Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534(1–2):15–20.
Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11.
Willis MS, Wians FH. The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clin Chim Acta. 2003;330:57–83.
Obermuller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, van der Vliet A, Valacchi G, et al. Lycopene inhibits the growth of normal human prostate epithelial cells invitro. J Nutr. 2003;133:3356–60.
Morgan DM. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol. 1998;79:179–83.
Nagao A. Oxidative conversion of carotenoids to retinoids and other products. J Nutr. 2004;134:237–40.
Holly LH, Leeanne F, Young, Keith RM. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med. 2005;230:171–9.
Chen L, Stacewicz-Sapuntzakis M, Duncan C. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–9.
Van Breemen RB. How do intermediate endpoint markers respond to lycopene in men with prostate cancer or benign prostate hyperplasia? J Nutr. 2005;135:2062–4.
Yang XW, Zhao J, Cui JR, Guo W. Studies on the biotransformation of escin Ia by human intestinal bacteria and the anti-tumor activities of desacylescin I. Beijing Da Xue Xue Bao. 2004;36:31–5.
Jayadev R, Jagan MRP, Malisetty VS, Chinthapally VR. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13:1392–8.
Hantz HL, Young LF, Martin KR. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med. 2005;230:171–9.
Dong X, Sanjay KS, Karen LL, Yan Z, Pamela H, Candace SJ, et al. Allyl isothiocynate a constituents of cruciferous vegetables inhibits proliferation of human prostate cancer cells by causing G2/M arrest and induce apoptosis. Carcinogenesis. 2003;24:891–7.
Lili T, Taiyi J, Xiangbin Z, Jia SW. Lycopene inhibits the growth of human androgen independent prostate cancer cells invitro and in BALB/c nude mice. Nutr Cancer. 2005;135:287–90.
Qin H, Morris N, Kang SJ, Li M, Tayo B, Lyon H, Hirschhorn J, Cooper RS, Zhu X. Interrogating local population structure for fine mapping in genome-wide association studies. Bioinformatics. 2010;26(23):2961–8.
Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95.
Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–18.
Manson MM. Cancer prevention—the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–8.
Kucuk O. Chemoprevention of prostate cancer. Cancer Metastasis Rev. 2002;21:111–24.
Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–6.
Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, et al. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomark Prevent. 1996;5:823–33.
Acknowledgments
We express gratitude to Dr. Hari, Sree Chithra Thirunal Institute of Medical Sciences, Trivandrum, India, for his help in the analysis of HPLC.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renju, G.L., Muraleedhara Kurup, G. & Bandugula, V.R. Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumor Biol. 35, 10747–10758 (2014). https://doi.org/10.1007/s13277-014-2339-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2339-5