Abstract
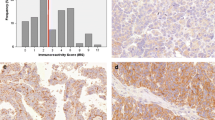
Replication factor C3 (RFC3) is an oncogene that can potentially predict prognosis in a variety of human cancers. RFC3 expression in ovarian carcinoma has not yet been determined. In this study, we evaluated the messenger RNA (mRNA) and protein expression levels of RFC3 in normal ovarian and ovarian carcinoma tissues using reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry, and Western blots (WB). Results showed that higher RFC3 mRNA and protein levels were detected in ovarian carcinoma tissues by RT-PCR and WB. High RFC3 expression was defined as positive staining in >70 % of each tumor cell. High RFC3 expression was detected in 28.1, 17.6, 11.1, and 5.0 % of invasive carcinomas, borderline tumors, cystadenomas, and in normal ovary cells, respectively. Overexpression of RFC3 was associated with later pN status (p = 0.001), pM status (p = 0.001), and advanced International Federation of Gynecology and Obstetrics (FIGO) stage (p = 0.012) in ovarian carcinomas. Univariate survival analyses showed that RFC3 overexpression was also associated with shortened patient survival (mean 7.7 months in tumors with RFC3 overexpression vs 92.9 months in tumors with normal RFC3 levels; p < 0.001). In multivariate analyses, RFC3 protein levels were a significant prognostic factor for ovarian carcinoma (p < 0.001). In conclusion, our findings suggest that RFC3 protein is an important and independent biomarker with prognostic implications for patients with ovarian carcinoma.




Similar content being viewed by others
References
Atlanta: American Cancer Society; American Cancer Society. Cancer Facts & Figures 2012. National Home Office; 2012.
Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43.
Gomez-Raposo C, Mendiola M, Barriuso J, Hardisson D, Redondo A. Molecular characterization of ovarian cancer by gene-expression profiling. Gynecol Oncol. 2010;118:88–92.
Ricciardelli C, Oehler MK. Diverse molecular pathways in ovarian cancer and their clinical significance. Maturitas. 2009;62:270–5.
Bast Jr RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28.
Vaughan S, Coward JI, Bast Jr RC, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–25.
Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29.
Lee SH, Kwong AD, Ishimi Y, Hurwitz J. Studies on the DNA elongation inhibitor and its proliferating cell nuclear antigen-dependent control in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989;86:4877–81.
Tsurimoto T, Stillman B. Purification of a cellular replication factor, RF-C, that is required for coordinated synthesis of leading and lagging strands during simian virus 40 DNA replication in vitro. Mol Cell Biol. 1989;9:609–19.
Shimada M, Okuzaki D, Tanaka S, et al. Replication factor C3 of Schizosaccharomyces pombe, a small subunit of replication factor C complex, plays a role in both replication and damage checkpoints. Mol Biol Cell. 1999;10:3991–4003.
Kim HS, Brill SJ. Rfc4 interacts with Rpa1 and is required for both DNA replication and DNA damage checkpoints in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:3725–37.
Fien K, Stillman B. Identification of replication factor C from Saccharomyces cerevisiae: a component of the leading-strand DNA replication complex. Mol Cell Biol. 1992;12:155–63.
Li X, Burgers PM. Molecular cloning and expression of the Saccharomyces cerevisiae RFC3 gene, an essential component of replication factor C. Proc Natl Acad Sci U S A. 1994;91:868–72.
Luckow B, Bunz F, Stillman B, Lichter P, Schutz G. Cloning, expression, and chromosomal localization of the 140-kilodalton subunit of replication factor C from mice and humans. Mol Cell Biol. 1994;14:1626–34.
Cullmann G, Fien K, Kobayashi R, Stillman B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4661–71.
Gray FC, MacNeill SA. The Schizosaccharomyces pombe rfc3+ gene encodes a homologue of the human hRFC36 and Saccharomyces cerevisiae Rfc3 subunits of replication factor C. Curr Genet. 2000;37:159–67.
Mossi R, Hubscher U. Clamping down on clamps and clamp loaders—the eukaryotic replication factor C. Eur J Biochem/FEBS. 1998;254:209–16.
Anderson LA, Perkins ND. The large subunit of replication factor C interacts with the histone deacetylase, HDAC1. J Biol Chem. 2002;277:29550–4.
Anderson LA, Perkins ND. Regulation of RelA (p65) function by the large subunit of replication factor C. Mol Cell Biol. 2003;23:721–32.
Koch HB, Zhang R, Verdoodt B, et al. Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle (Georgetown, Tex). 2007;6:205–17.
Arai M, Kondoh N, Imazeki N, et al. The knockdown of endogenous replication factor C4 decreases the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Liver Int: Off J Int Assoc Study Liver. 2009;29:55–62.
Jung HM, Choi SJ, Kim JK. Expression profiles of SV40-immortalization-associated genes upregulated in various human cancers. J Cell Biochem. 2009;106:703–13.
Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA. Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer. 2007;43:415–32.
Xiong S, Wang Q, Zheng L, Gao F, Li J. Identification of candidate molecular markers of nasopharyngeal carcinoma by tissue microarray and in situ hybridization. Med Oncol. 2011;28 Suppl 1:S341–8.
Lockwood WW, Thu KL, Lin L, et al. Integrative genomics identified RFC3 as an amplified candidate oncogene in esophageal adenocarcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18:1936–46.
Li Y, Yang HX, Luo RZ, et al. High expression of p300 has an unfavorable impact on survival in resectable esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:1531–8.
Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol. 2007;60:1112–6.
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: Cancer J Clin. 2011;61:212–36.
Badgwell D, Bast Jr RC. Early detection of ovarian cancer. Dis Markers. 2007;23:397–410.
Bast Jr RC. Early detection of ovarian cancer: new technologies in pursuit of a disease that is neither common nor rare. Trans Am Clin Climatol Assoc. 2004;115:233–47. discussion 47-8.
Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–19.
Mac SM, D'Cunha CA, Farnham PJ. Direct recruitment of N-myc to target gene promoters. Mol Carcinog. 2000;29:76–86.
Oliver TG, Grasfeder LL, Carroll AL, et al. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100:7331–6.
Spurgers KB, Gold DL, Coombes KR, et al. Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J Biol Chem. 2006;281:25134–42.
Vernell R, Helin K, Muller H. Identification of target genes of the p16INK4A-pRB-E2F pathway. J Biol Chem. 2003;278:46124–37.
Acknowledgments
This study was supported by the Natural Science Foundation of Guangdong Province (No. S2012010006150; No. S2012040006148) and Science and Technology Planning Project of Guangdong Province, China (No. 2011B031800056; No. 2011B031800276). Thanks are due to Forevergen Biosciences for assistance with the experiments and for valuable discussion. We are grateful to 91SCI Company for language editing assistance.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Huimin Shen and Muyan Cai contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 107 kb)
Rights and permissions
About this article
Cite this article
Shen, H., Cai, M., Zhao, S. et al. Overexpression of RFC3 is correlated with ovarian tumor development and poor prognosis. Tumor Biol. 35, 10259–10266 (2014). https://doi.org/10.1007/s13277-014-2216-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2216-2