Abstract
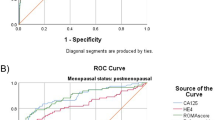
The aim of this study is to evaluate the diagnostic performance of human epididymis protein 4 (HE4), cancer antigen 125 (Ca125) and the risk of ovarian malignancy algorithm (ROMA) in discriminating ovarian cancer from other benign gynaecological diseases. Serum levels of HE4 and Ca125 were measured in 119 women with benign gynaecological diseases, 29 patients with primary ovarian cancer, 32 patients with ovarian cancer on chemotherapy treatment (18 of them with progressive disease), 6 patients treated and free of disease and 32 healthy women. Sensitivity, specificity, positive and negative predictive values and positive and negative likelihood ratios (LR±) were calculated. Receiver operator characteristic (ROC) curves were constructed, and the areas under the curve (AUC) were calculated. High serum levels for HE4, Ca125 and ROMA were observed in cancer patients. HE4 was elevated in 12.6 %, Ca125 in 21 % and ROMA in 9.2 % in the benign group, but HE4 was not elevated in endometriosis. The AUC values for HE4, Ca125 and ROMA were 0.92, 0.911 and 0.945 respectively. The sensitivity for discriminating ovarian cancer from benign gynaecological diseases was 86.2 % for HE4 and Ca125 and 93.1 % for ROMA. The specificity was 87.4, 78.9 and 90.7 % for HE4, Ca125 and ROMA. The overall positive likelihood ratio (LR+) was 6.84 for HE4, 4.1 for Ca125 and 10.01 for ROMA. In premenopausal women, LR + was 11.86 for HE4, 5.11 for ROMA and 2.02 for Ca125. HE4 might be significant in the differential diagnosis of ovarian cancer. HE4 seems to be superior to Ca125 in terms of diagnostic performance of all premenopausal women. ROMA could help to discriminate in cases with any doubt with a high diagnostic accuracy.

Similar content being viewed by others
References
Jemal A, Siegel RXJ, Sala E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300.
Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16 Suppl 1:11–7.
Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, et al. Surgery by consultant gynaecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106(3):589–98.
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumour markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54(12):e11–79.
Jacobs I, Bast Jr RC. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
Molina R, Ojeda B, Filella X, Borras G, Jo J, Mas E, et al. A prospective study of tumour markers CA 125 and CA 19.9 in patients with epithelial ovarian carcinomas. Tumour Biol. 1992;13:278–86.
Molina R, Auge JM, Escudero JM, Marrades R, Vinolas N, Carcereny E, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG 72.3 as tumour markers in patients with lung cancer: comparison with CYFRA 21–1, CEA, SCC and NSE. Tumour Biol. 2008;29:371–80.
Molina R, Auge JM, Bosch X, Escudero JM, Vinolas N, Marrades R, et al. Usefulness of serum tumour markers, including progastrin-releasing peptide, in patients with lung cancer: correlation with histology. Tumour Biol. 2009;30:121–9.
Markman M. The role of CA-125 in the management of ovarian cancer. Oncologist. 1997;2:6–9.
Molina R, Filella X, Bruix J, Mengual P, Bosch J, Calvet X, et al. Cancer antigen 125 in serum and ascitic fluid of patients with liver diseases. Clin Chem. 1991;37:1379–83.
Menon U, Gentry-Maharaj A, Hallet R, Ryan A, Burnell M, Sharma A et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer , and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009; 10 (4):327-340. available http://www.ncbi.nlm.nih.gov/pubmed/21642681
Patridge E, Kreimer AR, Greenlee RT, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113 (4):775-782. available http://www.ncbi.nlm.nih.gov/pubmed/19305319
Bast Jr RC, Klug TL, Schaetzl E, Lavin P, Niloff JM, Greber TF, et al. Monitoring human ovarian carcinoma with a combination of CA 125, CA 19–9, and carcinoembryonic antigen. Am J Obstet Gynecol. 1984;149:553–9.
Bast Jr RC, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumour markers: CA 125 and beyond. Int J Gynecol Cancer. 2005;15 Suppl 3:274–81.
van Dalen A, Favier J, Hallensleben E, Burges A, Stieber P, de Bruijn HW, et al. Significance of serum CA 125 and TPS antigen levels for determination of overall survival after three chemotherapy courses in ovarian cancer patients during long-term follow-up. Eur J Gynaecol Oncol. 2009;30:609–15.
Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–700.
Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–9.
Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumour biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8.
Havrilesky L, Whitehead C, Rubatt J, Cheek R, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110:374–82.
Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast Jr RC, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117:440–5.
Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. The utility of serum human epididymis protein 4 (HE4) in patients with malignant and non malignant diseases: comparison with CA 125. Clin Chem. 2011;57(11):1534–44.
Galgano M, Hampton G, Frierson HJ. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–53.
Moore RG, Brown AK, Craig Miller M, Badgwell D, Lu Z, Allard WJ, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110:196–201.
Hertlein L, Stieber P, Kirschenhofer A, Krocker K, Nagel D, Lenhard M, et al. Human epididymis protein (HE4) in benign and malignant diseases. Clin Chem Lab Med. 2012;50(12):2181–8.
Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Craig Miller M, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and Ca125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–6.
Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem. 2011;44(10–11):884–8. doi:10.1016/j.clinbiochem.2011.04.011.
Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315–9.
Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203:228–6.
Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–77.
National Institute for Health and Clinical Excellence (NICE): Guidance. Ovarian cancer: the recognition and initial management of ovarian cancer. National Collaborative Centre for Cancer (UK). Cardiff, UK: National Collaborative Centre for Cancer, 2011.
Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, et al. Use of Symptom Idex, Ca125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010;116:378–83.
Lenhard M, Stieber P, Hertlein L, Kirschenhofer A, Fürst S, Mayr D, et al. The diagnostic accuracy of two human epididymis protein 4 (HE4) testing systems in combination with CA 125 in the differential diagnosis of ovarian masses. Clin Chem Lab Med. 2011;49(12):2081–8.
Deeks JJ, Almant DG. Diagnostics test 4: likelihood ratios. BMJ. 2004;329(7458):168–9.
Molina R, Escudero JM, Augé JM, Filella X, Foj L, Torne A, et al. HE4 a novel tumour marker for ovarian cancer: comparison with Ca125 and ROMA algoritm in patients with gynaecological diseases. Tumor Biol. 2011;32:1087–95.
Park Y, Kim Y, Lee EY, Lee JH, Kim HS. Reference ranges for HE4 and CA125 in a large Asian population by automated assays and diagnostic performances for ovarian cancer. Int J Cancer. 2012;130:1136–44.
Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and Ca125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104:863–70.
Bandiera E, Romani C, Specchia C, Zanotti L, Galli C, Ruggeri G, et al. Serum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnositic and prognostic tools for epithelial ovarian cancer management. Cancer Epidemiol Biomarkers Prev. 2011;20:2496–506.
Yu S, Yang HJ, Xie SQ, Bao YX. Diagnostic value of HE4 for ovarian cancer: a meta-analysis. Clin Chem Lab Med. 2012;50(8):1439–46.
Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzolli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66:273–81.
Lin J, Qin J, Sangvatanakul V. Human epididymis protein 4 for differential diagnosis between benign gynaecologic disease and ovarian cancer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2013;167(1):81–5.
Li F, Ruxiu T, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and Ca125 in predicting epithelial ovarian cancer: A meta-analysis. BMC Cancer. 2012;12:258–76.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortiz-Muñoz, B., Aznar-Oroval, E., García, A.G. et al. HE4, Ca125 and ROMA algorithm for differential diagnosis between benign gynaecological diseases and ovarian cancer. Tumor Biol. 35, 7249–7258 (2014). https://doi.org/10.1007/s13277-014-1945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1945-6