Abstract
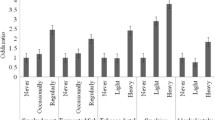
Nasopharyngeal carcinoma (NPC) is a rare cancer worldwide, but in India, NPC is uncommon in its subcontinent except in the north-eastern part of the country. NPC is thought to be caused by the combined effects of environmental carcinogens, genetic susceptibility and Epstein-Barr virus (EBV). This is the first study that aimed to examine the selected risk factors, mostly dietary, viral environmental, metabolic gene polymorphisms, mitochondrial DNA (mtDNA) copy number variation and their risk, in subjects who are highly prone to NPC in the ethnic groups of Northeast India, which has included cases, first-degree relatives and controls. The cases and controls were selected from three ethnic groups (Manipuri, Naga and Mizo) of Northeast India with high prevalence of NPC. This case–control family study includes 64 NPC patients, 88 first-degree relatives and 100 controls having no history of cancer. PCR-based detection was done for EBV–latent membrane protein 1 (LMP1) gene and glutathione S-transferase Mu 1 (GSTM1)–glutathione S-transferase theta 1 (GSTT1) polymorphism. A comparative ΔCt method was used for the determination of mtDNA content. An increased risk of 2.00–6.06-folds to NPC was observed with those who intake smoked meat and fish, salted fish and fermented fish; betel nut chewers; tobacco smokers; alcohol drinkers; and those who have kitchen inside the living room, glutathione S-transferase null genotype and EBV infection. The risk of NPC increased in cases with decreased mtDNA copy number (P trend = 0.007). A significant difference between GST null genotypes and EBV infection with mtDNA content was found in the cases (P < 0.0001). The understandings of environment–genetic risk factors and their role in the etiology of NPC are helpful as preventive measures and screening.



Similar content being viewed by others
References
Parkin DM, Whelan S, Ferlay J, Storm H. Cancer incidence in five continents. Lyon: IARC; 2005.
Curado MP, Edwards BK, Shin HR. Cancer incidence in five continents. Lyon, France: IARC; 2007.
Sharma TD, Singh TT, Laishram RS, Sharma LD, Sunita AK, Imchen LT. Nasopharyngeal carcinoma—a clinico-pathological study in a regional cancer centre of northeastern India. Asian Pac J Cancer Prev APJCP. 2011;12:1583–7.
Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22:117–26.
Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603.
Zhuo X, Cai L, Xiang Z, Li Q, Zhang X. GSTM1 and GSTT1 polymorphisms and nasopharyngeal cancer risk: an evidence-based meta-analysis. J Exp Clin Cancer Res CR. 2009;28:46.
Kumar S. Epidemiological and etiological factors associated with nasopharyngeal carcinoma. ICMR Bull. 2003;33:1–9.
Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomark Prev. 2006;15:1765–77.
Kiyohara C, Horiuchi T, Takayama K, Nakanishi Y. Genetic polymorphisms involved in carcinogen metabolism and DNA repair and lung cancer risk in a japanese population. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2012;7:954–62.
Jang J-H, Cotterchio M, Borgida A, Gallinger S, Cleary SP. Genetic variants in carcinogen-metabolizing enzymes, cigarette smoking and pancreatic cancer risk. Carcinogenesis. 2012;33:818–27.
Guo X, O’Brien SJ, Zeng Y, Nelson GW, Winkler CA. GSTM1 and GSTT1 gene deletions and the risk for nasopharyngeal carcinoma in han chinese. Cancer Epidemiol Biomark Prev. 2008;17:1760–3.
Mehrotra R, Yadav S. Oral squamous cell carcinoma: etiology, pathogenesis and prognostic value of genomic alterations. Indian J Cancer. 2006;43:60–6.
Yuan X, Li D, Zhao H, Jiang J, Wang P, Ma X, et al. Licochalcone A-induced human bladder cancer t24 cells apoptosis triggered by mitochondria dysfunction and endoplasmic reticulum stress. Biomed Res Int. 2013;2013:474272.
Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118.
Park JS, Sharma LK, Li H, Xiang R, Holstein D, Wu J, et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18:1578–89.
Ruwali M, Singh M, Pant MC, Parmar D. Polymorphism in glutathione S-transferases: susceptibility and treatment outcome for head and neck cancer. Xenobiotica. 2011;41:1122–30.
Mondal R, Ghosh SK, Talukdar FR, Laskar RS. Association of mitochondrial d-loop mutations with gstm1 and gstt1 polymorphisms in oral carcinoma: a case control study from northeast india. Oral oncol. 2012.
Mondal R, Ghosh SK, Choudhury JH, Seram A, Sinha K, Hussain M, et al. Mitochondrial DNA copy number and risk of oral cancer: a report from northeast india. PloS One. 2013;8:e57771.
Verma M, Naviaux RK, Tanaka M, Kumar D, Franceschi C, Singh KK. Meeting report: mitochondrial DNA and cancer epidemiology. Cancer Res. 2007;67:437–9.
Ghosh SK, Mondal R. Quick diagnosis of female genital tuberculosis using multiplex fast polymerase chain reaction in Southern Assam, India. Int J Gynaecol Obstet Official Organ Int Fed Gynaecol Obstet. 2012;118:72–3.
Mondal R, Ghosh SK, Talukdar FR, Laskar RS. Association of mitochondrial D-loop mutations with GSTM1 and GSTT1 polymorphisms in oral carcinoma: a case control study from northeast India. Oral Oncol. 2013;49:345–53.
Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–9.
Chelleng PK, Narain K, Das HK, Chetia M, Mahanta J. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Nat Med J India. 2000;13:6–8.
Pang LJ, Shao JY, Liang XM, Xia YF, Zeng YX. Mitochondrial DNA somatic mutations are frequent in nasopharyngeal carcinoma. Cancer Biol Ther. 2008;7:198–207.
Shao JY, Li YH, Gao HY, Mai HQ, Zhang Y, Guo X, et al. High frequency of common deletion (4981 bp) in mitochondrial DNA in nasopharyngeal carcinoma and its correlation with patient age and clinical stages. Cancer Biol Ther. 2004;3:1270–4.
Mondal R, Ghosh SK. Accumulation of mutations over the complete mitochondrial genome in tobacco-related oral cancer from northeast India. Mitochondrial DNA. 2013.
Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX, Liu WS, et al. Traditional cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in guangdong, china. BMC Cancer. 2010;10:446.
Old LJ, Boyse EA, Oettgen HF, Harven ED, Geering G, Williamson B, et al. Precipitating antibody in human serum to an antigen present in cultured Burkitt’s lymphoma cells. Proc Natl Acad Sci U S A. 1966;56:1699–704.
Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer cell. 2004;5:423–8.
Hu LF, Zabarovsky ER, Chen F, Cao SL, Ernberg I, Klein G, et al. Isolation and sequencing of the epstein-barr virus BNLF-1 gene (LMP1) from a chinese nasopharyngeal carcinoma. J Gen Virol. 1991;72(Pt 10):2399–409.
Lin JC, Cherng JM, Lin HJ, Tsang CW, Liu YX, Lee SP. Amino acid changes in functional domains of latent membrane protein 1 of epstein-barr virus in nasopharyngeal carcinoma of southern china and taiwan: prevalence of an HLA A2-restricted ‘epitope-loss variant’. J Gen Virol. 2004;85:2023–34.
Eliopoulos AG, Blake SM, Floettmann JE, Rowe M, Young LS. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999;73:1023–35.
Higuchi M, Kieff E, Izumi KM. The Epstein-Barr virus latent membrane protein 1 putative janus kinase 3 (JAK3) binding domain does not mediate JAK3 association or activation in B-lymphoma or lymphoblastoid cell lines. J Virol. 2002;76:455–9.
Nicholson LJ, Hopwood P, Johannessen I, Salisbury JR, Codd J, Thorley-Lawson D, et al. Epstein-barr virus latent membrane protein does not inhibit differentiation and induces tumorigenicity of human epithelial cells. Oncogene. 1997;15:275–83.
Dawson CW, Eliopoulos AG, Blake SM, Barker R, Young LS. Identification of functional differences between prototype Epstein-Barr virus-encoded LMP1 and a nasopharyngeal carcinoma-derived LMP1 in human epithelial cells. Virology. 2000;272:204–17.
Pandya J, Walling DM. Oncogenic activity of Epstein-Barr virus latent membrane protein 1 (LMP-1) is down-regulated by lytic LMP-1. J Virol. 2006;80:8038–46.
Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993;90:8479–83.
Hickish T, Robertson D, Clarke P, Hill M, di Stefano F, Clarke C, et al. Ultrastructural localization of bhrf1: an Epstein-Barr virus gene product which has homology with Bcl-2. Cancer Res. 1994;54:2808–11.
Li LY, Liu MY, Shih HM, Tsai CH, Chen JY. Human cellular protein VRK2 interacts specifically with Epstein-Barr virus BHRF1, a homologue of Bcl-2, and enhances cell survival. J Gen Virol. 2006;87:2869–78.
Lu ZX, Ye M, Yan GR, Li Q, Tang M, Lee LM, et al. Effect of EBV LMP1 targeted dnazymes on cell proliferation and apoptosis. Cancer Gene Ther. 2005;12:647–54.
Kawanishi M, Tada-Oikawa S, Kawanishi S. Epstein-Barr virus BHRF1 functions downstream of bid cleavage and upstream of mitochondrial dysfunction to inhibit trail-induced apoptosis in bjab cells. Biochem Biophys Res Commun. 2002;297:682–7.
Moaven O, Raziee HR, Sima HR, Ganji A, Malekzadeh R, A’Rabi A, et al. Interactions between glutathione-S-transferase M1, T1 and P1 polymorphisms and smoking, and increased susceptibility to esophageal squamous cell carcinoma. Cancer Epidemiol. 2010;34:285–90.
Vrana D, Pikhart H, Mohelnikova-Duchonova B, Holcatova I, Strnad R, Slamova A, et al. The association between glutathione S-transferase gene polymorphisms and pancreatic cancer in a central European Slavonic population. Mutat Res. 2009;680:78–81.
Ahmad ST, Arjumand W, Seth A, Kumar Saini A, Sultana S. Impact of glutathione transferase M1, T1, and P1 gene polymorphisms in the genetic susceptibility of north Indian population to renal cell carcinoma. DNA Cell Biol. 2012;31:636–43.
Bendjemana K, Abdennebi M, Gara S, Jmal A, Ghanem A, Touati S, et al. [Genetic polymorphism of gluthation-S transferases and N-acetyl transferases 2 and nasopharyngeal carcinoma: the Tunisia experience]. Bull Cancer. 2006;93:297–302.
Jiang Y, Li N, Dong P, Zhang N, Sun Y, Han M, et al. Polymorphisms in GSTM1, GSTTI and GSTP1 and nasopharyngeal cancer in the east of China: a case-control study. Asian Pac J Cancer Prev APJCP. 2011;12:3097–100.
Sun ZF, Zhang J, Xu HM, Wang GL, Dong P. Association between gstm1 polymorphism and nasopharyngeal cancer susceptibility: a meta-analysis. Asian Pac J Cancer Prev APJCP. 2012;13:5817–21.
Lee HC, Yin PH, Yu TN, Chang YD, Hsu WC, Kao SY, et al. Accumulation of mitochondrial DNA deletions in human oral tissues—effects of betel quid chewing and oral cancer. Mutat Res. 2001;493:67–74.
Jiang WW, Rosenbaum E, Mambo E, Zahurak M, Masayesva B, Carvalho AL, et al. Decreased mitochondrial DNA content in posttreatment salivary rinses from head and neck cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:1564–9.
Mondal R, Ghosh SK. Accumulation of mutations over the complete mitochondrial genome in tobacco-related oral cancer from northeast india. Mitochondrial DNA. 2013;24:432–9.
Acknowledgments
Our humble acknowledgement goes to the Department of Biotechnology (DBT), Govt. of India for providing infra-structural facilities for conducting research on Cancer. Our sincere thanks go to Naga Hospital in Kohima, Nagaland, RIMS Imphal, Manipur and Civil hospital Aizawl, Mizoram for the collected samples and data.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
SK Ghosh, AS Singh and R Mondal equally contributed to this study.
Rights and permissions
About this article
Cite this article
Ghosh, S.K., Singh, A.S., Mondal, R. et al. Dysfunction of mitochondria due to environmental carcinogens in nasopharyngeal carcinoma in the ethnic group of Northeast Indian population. Tumor Biol. 35, 6715–6724 (2014). https://doi.org/10.1007/s13277-014-1897-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1897-x