Abstract
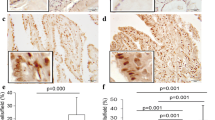
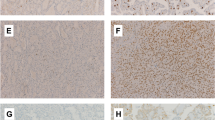
Studies have shown that SCC-S2 can be detected in cancer cells, but its relation with thyroid cancer remains uncertain. In the current study, we investigated SCC-S2 expression in thyroid cancer from the immune cell perspective and tumor tissue perspective. Levels of SCC-S2 in CD4+ T cells, CD8+ T cells, monocytes, natural killer (NK) T cells, tumor tissues, and adjacent noncancerous thyroid tissues were tested by real-time reverse transcription PCR and Western blot. Results revealed that mRNA and protein levels of SCC-S2 were significantly increased in peripheral CD4+ (mRNA, 1.90-fold; protein, 1.55-fold) and CD8+ T cells (mRNA, 2.37-fold; protein, 1.72-fold) but not monocytes and NKT cells in patients than in healthy donors. Further elevated mRNA level but not protein expression was observed in tumor-infiltrating CD4+ T cells, whereas both mRNA level and protein expression were further increased in tumor-infiltrating CD8+ T cells. Also, mRNA and protein levels of SCC-S2 in thyroid tissues were significantly elevated than those in adjacent noncancerous thyroid tissues. Moreover, patients with cervical lymph node metastasis presented clearly higher mRNA and protein expression of SCC-S2 compared to those without cervical lymph node metastasis (p < 0.05). These results suggest that SCC-S2 may play roles in affecting both immune cells and tumor cells in the thyroid and may indicate a novel pathway for understanding the pathogenesis of the disease.





Similar content being viewed by others
References
Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306.
Roger PP, van Staveren WC, Coulonval K, Dumont JE, Maenhaut C. Signal transduction in the human thyrocyte and its perversion in thyroid tumors. Mol Cell Endocrinol. 2010;321:3–19.
Freundt EC, Bidere N, Lenardo MJ. A different TIPE of immune homeostasis. Cell. 2008;133:401–2.
Filkor K, Hegedus Z, Szasz A, Tubak V, Kemeny L, Kondorosi E, Nagy I. Genome wide transcriptome analysis of dendritic cells identifies genes with altered expression in psoriasis. PloS one. 2013;8:e73435.
Laliberte B, Wilson AM, Nafisi H, Mao H, Zhou YY, Daigle M, Albert PR. TNFAIP8: a new effector for Galpha(i) coupling to reduce cell death and induce cell transformation. J. Cell. Physiol. 2010;225:865–74.
Li A, Chen Y, Zhao X, Niu Y, Cong P, Zhang Z, Chen W, Jiang W, Mo D. Characterization and transcriptional regulation analysis of the porcine TNFAIP8L2 gene. Mol. Gen. Genomics. 2010;284:185–95.
Liu T, Gao H, Chen X, Lou G, Gu L, Yang M, Xia B, Yin H. TNFAIP8 as a predictor of metastasis and a novel prognostic biomarker in patients with epithelial ovarian cancer. Br. J. Cancer. 2013;109:1685–92.
Luan YY, Yao YM, Sheng ZY. The tumor necrosis factor-alpha-induced protein 8 family in immune homeostasis and inflammatory cancer diseases. J. Biol. Regul. Homeost. Agents. 2013;27:611–19.
Romanuik TL, Ueda T, Le N, Haile S, Yong TM, Thomson T, Vessella RL, Sadar MD. Novel biomarkers for prostate cancer including noncoding transcripts. Am. J. Pathol. 2009;175:2264–76.
Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–26.
Takahashi Y, Tsuruta S, Honda A, Fujiwara Y, Satoh M, Yasutake A. Effect of dental amalgam on gene expression profiles in rat cerebrum, cerebellum, liver and kidney. J. Toxicol. Sci. 2012;37:663–6.
Wang L, Song Y, Men X. Variance of TNFAIP8 expression between tumor tissues and tumor-infiltrating CD4+ and CD8+ T cells in non-small cell lung cancer. Tumour Biol. 2013. doi:10.1007/s13277-013-1307-9.
Wang LY, Fan YC, Zhao J, Gao S, Sun FK, Han J, Yang Y, Wang K. Elevated expression of tumour necrosis factor-alpha-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral blood mononuclear cells is associated with disease progression of acute-on-chronic hepatitis B liver failure. J. Viral Hepat. 2014;21:64–73.
Woodward MJ, de Boer J, Heidorn S, Hubank M, Kioussis D, Williams O, Brady HJ. Tnfaip8 is an essential gene for the regulation of glucocorticoid-mediated apoptosis of thymocytes. Cell Death Differ. 2010;17:316–23.
Zhang S, Zhang Y, Wei X, Zhen J, Wang Z, Li M, Miao W, Ding H, Du P, Zhang W et al. Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. Biochim. Biophys. Acta 2010;1802:1078–86.
Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat. Struct. Mol. Biol. 2009;16:89–90.
Zhang Y, Chen MB, Zhou XY, Hong XN. Lymphotoxin alpha (LTA) polymorphism is associated with prognosis of non-Hodgkin's lymphoma in a Chinese population. PloS one. 2013;8:e66411.
Zhang Y, Wang MY, He J, Wang JC, Yang YJ, Jin L, Chen ZY, Ma XJ, Sun MH, Xia KQ et al. Tumor necrosis factor-alpha induced protein 8 polymorphism and risk of non-Hodgkin's lymphoma in a Chinese population: a case-control study. PloS one. 2012; 7:e37846.
Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 2006;36:1892–1903.
Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J. Immunol. 2008;180:2967–80.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25 T cells. Blood. 2007;110:2537–44.
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res 2009;69:5522–30.
Meier P, Finch A, Evan G. Apoptosis in development. Nature 2000;407:796–801.
Acknowledgements
We thank Lingcheng Wang, Yinghua Song, and Xuelin Men for their great contributions in directing experiments and writing paper.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, D., Zhu, YQ., Guan, LL. et al. Upregulation of SCC-S2 in immune cells and tumor tissues of papillary thyroid carcinoma. Tumor Biol. 35, 4331–4337 (2014). https://doi.org/10.1007/s13277-013-1568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1568-3