Abstract
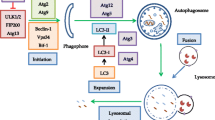
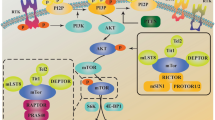
Radiotherapy is one of the main treatments for clinical cancer therapy. However, its application was limited due to lack of radiosensitivity in some cancers. Trichostatin A (TSA) is a classic histone deacetylases inhibitor (HDACi) that specifically inhibits the biochemical functions of HDAC and is demonstrated to be an active anticancer drug. However, whether it could sensitize colon cancer to radiation is not clear. Our results showed that TSA enhanced the radiosensitivity of colon cancer cells as determined by CCK-8 and clonogenic survival assay. Moreover, apoptotic cell death induced by radiation was enhanced by TSA treatment. Additionally, TSA also induced autophagic response in colon cancer cells, while autophagy inhibition led to cell apoptosis and enhanced the radiosensitivity of colon cancer cells. Our data suggested that inhibition of cytoprotective autophagy sensitizes cancer cell to radiation, which might be further investigated for clinical cancer radiotherapy.





Similar content being viewed by others
References
Liu S et al. Randomized, controlled phase II study of post-surgery radiotherapy combined with recombinant adenoviral human p53 gene therapy in treatment of oral cancer. Canc Gene Ther. 2013;20(6):375–8.
Lövey J et al. Radiosensitivity of human prostate cancer cells can be modulated by inhibition of 12-lipoxygenase. Cancer Lett. 2013;335(2):495–501.
Benzina S et al. High-LET radiation combined with oxaliplatin induce autophagy in U-87 glioblastoma cells. Cancer Lett. 2008;264(1):63–70.
Weichert W et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14(6):1669–77.
Weichert W et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9(2):139–48.
Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280(2):168–76.
Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–99.
Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–84.
Carafa V, Nebbioso A, Altucci L. Histone deacetylase inhibitors: recent insights from basic to clinical knowledge & patenting of anti-cancer actions. Recent Pat Anticancer Drug Discov. 2011;6(1):131–45.
Mottet D, Castronovo V. Histone deacetylases: anti-angiogenic targets in cancer therapy. Curr Cancer Drug Targets. 2010;10(8):898–913.
Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin Cancer Res. 2009;15(12):3970–7.
Gore SD et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66(12):6361–9.
Caron C, Boyault C, Khochbin S. Regulatory cross-talk between lysine acetylation and ubiquitination: role in the control of protein stability. Bioessays. 2005;27(4):408–15.
Fantin VR et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68(10):3785–94.
Oh M, Choi IK, Kwon HJ. Inhibition of histone deacetylase1 induces autophagy. Biochem Biophys Res Commun. 2008;369(4):1179–83.
Gammoh N et al. Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl Acad Sci U S A. 2012;109(17):6561–5.
Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17(10):839–49.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36.
Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19(5):698–711.
Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286–98.
Liu Y, He G, Wang Y, Guan X, Pang X, Zhang B. MCM-2 is a therapeutic target of Trichostatin A in colon cancer cells. Toxicol Lett. 2013;221(1):23–30.
Rhodes LV et al. The histone deacetylase inhibitor trichostatin A alters microRNA expression profiles in apoptosis-resistant breast cancer cells. Oncol Rep. 2012;27(1):10–6.
Frew AJ et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci U S A. 2008;105(32):11317–22.
Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J Clin Oncol. 2007;26:4051–6.
Folkvord S et al. Radiosensitization by SAHA in experimental colorectal carcinoma models—in vivo effects and relevance of histone acetylation status. Int J Radiat Oncol Biol Phys. 2009;74(2):546–52.
Ree AH et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 2010;11(5):459–64.
Chowdhury S et al. Histone deacetylase inhibitor belinostat represses survivin expression through reactivation of transforming growth factor beta (TGFbeta) receptor II leading to cancer cell death. J Biol Chem. 2011;286(35):30937–48.
Kondo Y et al. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726–34.
Livesey KM et al. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs. 2009;10(12):1269–79.
Sy LK et al. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 2008;68(24):10229–37.
Akar U et al. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4(5):669–79.
Funderburk SF, Wang QJ, Yue Z. The Beclin 1–VPS34 complex—at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20(6):355–62.
Dai ZJ et al. Antitumor effects of rapamycin in pancreatic cancer cells by inducing apoptosis and autophagy. Int J Mol Sci. 2013;14(1):273–85.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 81272497).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 657 kb)
Rights and permissions
About this article
Cite this article
He, G., Wang, Y., Pang, X. et al. Inhibition of autophagy induced by TSA sensitizes colon cancer cell to radiation. Tumor Biol. 35, 1003–1011 (2014). https://doi.org/10.1007/s13277-013-1134-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1134-z