Abstract
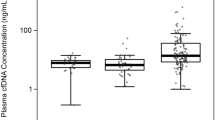
The aim of this study was to evaluate the diagnostic and potential prognostic value of cell-free plasma DNA (CF-pDNA) in patients with suspected or histologically proven prostate cancer (PCa). We included 133 men with a diagnosis of PCa and 33 controls. PCa patients had blood samples prospectively drawn every 3 months for 2 years. CF-pDNA was measured by spectrophotometry. Considering a cut-off value of 140 ng/mL of CF-pDNA the area under the curve was of 0.824(0.757–0.879 with a sensitivity = 66.2 % and a specificity = 87.9 %) and the positive and negative likelihood ratio were of 5.46 and 0.39, respectively. CF-pDNA tends to decrease slightly and return to baseline values in about a week after biopsy. There was no statistical significant correlation between CF-pDNA levels at study entry with PSA, Gleason score, stage and biochemical recurrence free survival (BRFS). However, with a mean follow-up of 13.5 months, we could observe a significant shorter BRFS for patients with at least one value above 140 ng/mL of CF-pDNA during follow-up (p = 0.048). CF-pDNA is a potentially valuable biomarker for PCa diagnosis and a potential tool for the follow-up of patients with PCa.



Similar content being viewed by others
References
NIH/SEER database (2013) SEER Stat Fact Sheets: prostate. http://seer.cancer.gov/statfacts/html/prost.html#incidence-mortality. Accessed 29 Mar 2013.
Serpa-Neto A, Tobias-Machado M, Wroclawski ML, Akerman M, Pompeo ACL, Del Giglio A. Descriptive study of prostate cancer mortality in the State of São Paulo, from 1980 to 2007. Einstein. 2010;8:433–6.
Wroclawski ML, Kuk C, Finelli A, Fleshner NE, Zlotta AR. Chemoprevention of prostate cancer: is there evidence from clinical trials? Clin Invest. 2011;1:1257–68.
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71.
Guidelines On-line—SBU Nacional. Módulo 4—Urooncologia. http://sbu.org.br/pdf/guideline/4/CaProstata.pdf. Accessed 29 Jan 2013.
Harvey P, Basuita A, Endersby D, Curtis B, Iacovidou A, Walker M. A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol. 2009;9:14.
Mosharafa AA, Torky MH, El Said WM, Meshref A. Rising incidence of acute prostatitis following prostate biopsy: fluoroquinolone resistance and exposure is a significant risk factor. Urology. 2011;78:511–4.
Shapiro B, Chakrabarty M, Cohn EM, Leon SA. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer. 1983;51:2116–20.
Sozzi G, Conte D, Mariani L, Lo Vullo S, Roz L, Lombardo C, et al. Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res. 2001;61:4675–8.
Umetani N, Kim J, Hiramatsu S, Reber HA, Hines OJ, Bilchik AJ, et al. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin Chem. 2006;52:1062–9.
Trejo-Becerril C, Pérez-Cárdenas E, Treviño-Cuevas H, Taja-Chayeb L, García-López P, Segura-Pacheco B, et al. Circulating nucleosomes and response to chemotherapy: an in vitro, in vivo, and clinical study on cervical cancer patients. Int J Cancer. 2003;104:663–8.
Chang HW, Lee SM, Goodman SN, Singer G, Cho SK, Sokoll LJ, et al. Assessment of plasma DNA levels, allelic imbalance, and CA 125 as diagnostic tests for cancer. J Natl Cancer Inst. 2002;94:1697–703.
Huang ZH, Li LH, Hua D. Quantitative analysis of plasma circulating DNA at diagnosis and during follow-up of breast cancer patients. Cancer Lett. 2006;243:64–70.
Ellinger J, Wittkamp V, Albers P, Perabo FG, Mueller SC, von Ruecker A, et al. Cell-free circulating DNA: diagnostic value in patients with testicular germ cell cancer. J Urol. 2009;181:363–71.
Ellinger J, Bastian PJ, Ellinger N, Kahl P, Perabo FG, Büttner R, et al. Apoptotic DNA fragments in serum of patients with muscle invasive bladder cancer: a prognostic entity. Cancer Lett. 2008;264:274–80.
Jung K, Stephan C, Lewandowski M, Klotzek S, Jung M, Kristiansen G, et al. Increased cell-free DNA in plasma of patients with metastatic spread in prostate cancer. Cancer Lett. 2004;205:173–80.
Schwarzenbach H, Alix-Panabières C, Müller I, Letang N, Vendrell JP, Rebillard X, et al. Cell-free tumor DNA in blood plasma as a marker for circulating tumor cells in prostate cancer. Clin Cancer Res. 2009;15:1032–8.
Chun FK, Müller I, Lange I, Friedrich MG, Erbersdobler A, Karakiewicz PI, et al. Circulating tumor-associated plasma DNA represents an independent and informative predictor of prostate cancer. BJU Int. 2006;98:544–8.
Serpa Neto A, Wroclavski ML, Pinto JLF, Marsicano SR, Delgado PO, Coelho PG, et al. Methodological standardization for the extraction of free DNA in plasma of peripheral blood. J Cancer Sci Ther. 2012. doi:10.4172/1948-5956.S5-005.
Stephenson RA. Prostate cancer trends in the era of prostate-specific antigen. An update of incidence, mortality, and clinical factors from the SEER database. Urol Clin North Am. 2002;29:173–81.
Delgado PO, Alves BC, de Sousa GF, Kuniyoshi RK, Wroclavski ML, Del Giglio A, et al. Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumour Biol. 2013;34:983–6.
Levy DA, Jones JS. Management of rising prostate specific antigen after a negative biopsy. Curr Urol Rep. 2011;12:197–202.
Allen D, Butt A, Cahill D, Wheeler M, Popert R, Swaminathan R. Role of cell-free plasma DNA as a diagnostic marker for prostate cancer. Ann NY Acad Sci. 2004;1022:76–80.
Papadopoulou E, Davilas E, Sotiriou V, Koliopanos A, Aggelakis F, Dardoufas K, et al. Cell-free DNA and RNA in plasma as a new molecular marker for prostate cancer. Oncol Res. 2004;14:439–45.
Altimari A, Grigioni AD, Benedettini E, Gabusi E, Schiavina R, Martinelli A, et al. Diagnostic role of circulating free plasma DNA detection in patients with localized prostate cancer. Am J Clin Pathol. 2008;129:756–62.
Bastian PJ, Palapattu GS, Yegnasubramanian S, Lin X, Rogers CG, Mangold LA, et al. Prognostic value of preoperative serum cell-free circulating DNA in men with prostate cancer undergoing radical prostatectomy. Clin Cancer Res. 2007;13:5361–7.
Ellinger J, Bastian PJ, Haan KI, Heukamp LC, Buettner R, Fimmers R, et al. Noncancerous PTGS2 DNA fragments of apoptotic origin in sera of prostate cancer patients qualify as diagnostic and prognostic indicators. Int J Cancer. 2008;122:138–43.
Boddy JL, Gal S, Malone PR, Harris AL, Wainscoat JS. Prospective study of quantitation of plasma DNA levels in the diagnosis of malignant versus benign prostate disease. Clin Cancer Res. 2005;11:1394–9.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wroclawski, M.L., Serpa-Neto, A., Fonseca, F.L.A. et al. Cell-free plasma DNA as biochemical biomarker for the diagnosis and follow-up of prostate cancer patients. Tumor Biol. 34, 2921–2927 (2013). https://doi.org/10.1007/s13277-013-0854-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0854-4