Abstract
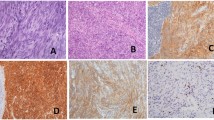
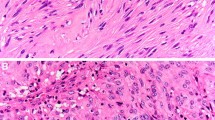
The purpose of this study is to detect the clinicopathology of gastrointestinal stromal tumors (GISTs) occurring synchronously with gastric adenocarcinomas and to unveil the potential underlying relationship between the synchronous GIST and gastric adenocarcinoma. This study included 15 patients with incidental GISTs found during operations for gastric adenocarcinoma and 30 patients who underwent gastrectomy for gastric cancer without discovering GIST between January 2005 and December 2010 at the Beijing Cancer Institute. We collected the clinicopathological data and analyzed the KIT/PDGFRA mutational status of GISTs, corresponding gastric adenocarcinoma specimens, and the normal tissue around the cancer lesions. Additionally, as a control group, the mutational status of the patients with gastric adenocarcinoma and no other tumors was assayed. Overall, 18 GISTs were found in 15 gastric adenocarcinoma patients. Multiple GIST lesions were found in three cases (20 %). The patients’ age ranged from 46 to 85 years, with an average of 67.6 years. The average size of the GISTs was 0.85 cm. All mesenchymal lesions showed low proliferative activity, were of low or very low risk, and were identified as CD117-positive by immunostaining. In GIST lesions, mutations in KIT were detected in 7 out of 13 cases, and of these mutations, 6 were found in exon 11 (46.2 %), and 1 was found in exon 9 (7.7 %). A total of five deletions and one point mutation were in exon 11, and one insertion was in exon 9. Mutations were not detected in exon 17 or 13 of KIT. There was no remarkable mutation analyzed in the gastric adenocarcinoma lesions or normal tissues from either the test or control groups. Clinicopathological profiles and molecular analysis of KIT/PDGFRA showed no obvious relationship between gastric cancer and GISTs in tumor genesis, such as similar oncogene mutations.

Similar content being viewed by others
References
Bachet JB, et al. Prognosis and predictive value of KIT exon 11 deletion in GISTs. Br J Cancer. 2009;101(1):7–11.
Tzen CY, Wang MN, Mau BL. Spectrum and prognostication of KIT and PDGFRA mutation in gastrointestinal stromal tumors. Eur J Surg Oncol. 2008;34(5):563–8.
Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53(3):245–66.
Blay JY, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16(4):566–78.
Gastric Cancer Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health. Chinese guidelines for diagnosis and treatment of gastric cancer (2011 edition). Transl Gastrointest Cancer. 2012;1:103–14.
Hu B, et al. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–61.
Zhao X, Yue C. Gastrointestinal stromal tumor. J Gastrointest Oncol. 2012;3(3):189–208.
Fletcher CD, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81–9.
Gomes A, et al. Molecular analysis of c-Kit and PDGFRA in GISTs diagnosed by EUS. Am J Clin Pathol. 2007;127(1):89–96.
Fletcher C. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–65.
Burger H, et al. Activating mutations in c-KIT and PDGFRalpha are exclusively found in gastrointestinal stromal tumors and not in other tumors overexpressing these imatinib mesylate target genes. Cancer Biol Ther. 2005;4(11):1270–4.
Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15(5):453–6.
Schneider BJ, et al. Phase II trial of imatinib maintenance therapy after irinotecan and cisplatin in patients with c-Kit-positive, extensive-stage small-cell lung cancer. Clin Lung Cancer. 2010;11(4):223–7.
Huh WK, et al. Efficacy and safety of imatinib mesylate (Gleevec) and immunohistochemical expression of c-Kit and PDGFR-beta in a Gynecologic Oncology Group Phase Il Trial in women with recurrent or persistent carcinosarcomas of the uterus. Gynecol Oncol. 2010;117(2):248–54.
Nilsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103(4):821–9.
Monges G, et al. The estimated incidence of gastrointestinal stromal tumors in France. Results of PROGIST study conducted among pathologists. Bull Cancer. 2010;97(3):E16–22.
Espinosa I, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32(2):210–8.
Goettsch WG, et al. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer. 2005;41(18):2868–72.
Tzen CY, et al. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational analyses. Dig Dis Sci. 2007;52(3):792–7.
Ruka W, et al. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST). Med Sci Monit. 2004;10(8):LE13–4.
Kövér E, Faluhelyi Z, Bogner B, Kalmár K, Horváth G, Tornóczky T. Kettôs tumorok a gasztrointesztinális traktusban: szinkron és metakron stromális (GIST) és epiteliális/neuroendokrin daganatok. Magyar Onkológia. 2004;48(4):315–21.
Au WY, Ho KM, Shek TW. Papillary renal cell carcinoma and gastrointestinal stromal tumor: a unique association. Ann Oncol. 2004;15(5):843–4.
Wronski M, et al. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12(33):5360–2.
Agaimy A, et al. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23(2):120–9.
Strandberg L, et al. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J Clin Immunol. 2008;28(3):220–31.
Abraham SC, et al. "Seedling" mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31(11):1629–35.
Kawanowa K, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527–35.
Rossi S, et al. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am J Surg Pathol. 2010;34(10):1480–91.
Agaimy A, Wunsch PH, Hofstaedter F, Blaszyk H, Rummele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31(1):113–20.
Agaimy A, et al. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol. 2008;32(6):867–73.
Agaimy A, Wunsch PH. Sporadic Cajal cell hyperplasia is common in resection specimens for distal oesophageal carcinoma. A retrospective review of 77 consecutive surgical resection specimens. Virchows Arch. 2006;448(3):288–94.
Corless CL, et al. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160(5):1567–72.
Kawanowa K, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Human Pathol. 2006;37(12):1527–35.
Chetty R. Small and microscopically detected gastrointestinal stromal tumours: an overview. Pathology. 2008;40(1):9–12.
Ogasawara N, et al. Frequent c-Kit gene mutations not only in gastrointestinal stromal tumors but also in interstitial cells of Cajal in surrounding normal mucosa. Cancer Lett. 2005;230(2):199–210.
Almaca J, et al. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284(42):28571–8.
Agaimy A, Wuensch PH. Gastrointestinal stromal tumours in patients with other-type cancer: a mere coincidence or an etiological association? A study of 97 GIST cases. Z Gastroenterol. 2005;43(9):1025–30.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yan Yan, Ziyu Li, and Yiqiang Liu contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 34 kb)
Rights and permissions
About this article
Cite this article
Yan, Y., Li, Z., Liu, Y. et al. Coexistence of gastrointestinal stromal tumors and gastric adenocarcinomas. Tumor Biol. 34, 919–927 (2013). https://doi.org/10.1007/s13277-012-0627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0627-5