Abstract
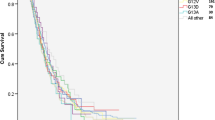
The efficacy of epidermal growth factor-targeting therapies has been found to be limited in tumors with the wild-type K-RAS gene, suggesting a predictive value of K-RAS gene analysis in tumoral response. However, the prognostic value of K-RAS is controversial. This study included patients diagnosed with metastatic colorectal cancer. The presence of K-RAS mutations was analyzed, and the tumors positive for a K-RAS mutation were further analyzed to identify the mutation type. Similarly, the following clinical and pathological variables were also collected. The study was composed of 53.3 % of patients with wild-type K-RAS and 46.7 % of patients with mutated K-RAS (mutated codon 12 was the most frequent). With a mean follow-up of 15 months (range, 1–45), the median survival of patients with wild-type K-RAS was 31.6 months. The median survival was 24.8 months for patients with K-RAS mutated in codon 12 and 17.8 months for patients with mutated codon 13 (p = 0.37). In a univariate analysis, K-RAS was associated with stage IV at diagnosis (p < 0.005). When K-RAS was mutated, a lower overall survival was observed in cases of G→A transition compared with G→T transversion (19.5 vs. 24.2 months, respectively; p = 0.47). When the amino acid change resulted in an acidic substitution, survival was lower, but it increased when the substitution resulted in a polar or nonpolar amino acid (19.5 vs. 23.2 vs. 24.4 months, p = 0.79). The type of K-RAS mutation or amino acid changes may have prognostic implications in metastatic colon cancer patients. Further research is needed in patients treated in prospective controlled trials.



Similar content being viewed by others
References
Vogelstein B, Fearon E, et al. Genetic alteration during colorectal tumor development. N Engl J Med. 1998;319:525–32.
Bos JL, Fearon EL, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature Res. 1990;50:7717–22.
Neumann J, Zeindl-Eberhart E, Kirchner T, et al. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res. 2009;205:858–62.
Heinemann V, Stintzing S, Kirchner T, et al. Clinical relevance of EGFR—and KRAS—status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev. 2009;35:262–71.
Bokemeyer C, Igor B, Anatoly M, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71.
Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME Study. J Clin Oncol. 2010;28:4697–705.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-RAS mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Qiu LX, Mao C, Zhang J, et al. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. EJC. 2010;46:2781–7.
De Roock W, Jonker DJ, Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–20.
Span M, Moerkerk P, Goeij A, et al. A detailed analysis of K-RAS point mutations in relation to tumor progression and survival in colorectal cancer patients. Int J Cancer. 1996;69:241–5.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds.) AJCC cancer staging handbook. 6th Printing; 2010, XIV, 730 p. 130 illu.
Linardou H, Briasoulis E, Dahabreh IJ, et al. All about KRAS for clinical oncology practice: gene profile, clinical implications and laboratory recommendations for somatic mutational testing in colorectal cancer. Cancer Treat Rev. 2011;37:221–33.
Esteller M, et al. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304.
Andreyev HJ, Norman R, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL” study. J Natl Cancer Inst. 1998;90:675–84.
Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:1–10.
Dix BR, et al. The common molecular genetic alterations in Dukes' B and C colorectal carcinomas are not short-term prognostic indicators of survival. Int J Cancer. 1994;59:747–51.
Roth A, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74.
Deschoolmeester V, Boeckx C, Baay M, et al. KRAS mutation detection and prognostic potential in sporadic colorectal cancer using high-resolution melting analysis. Br J Cancer. 2010;103:1627–36.
Poehlmann A, Kuester D, Meyer F, et al. KRAS mutation detection in colorectal cancer using the pyrosequencing technique. Pathol Res Pract. 2007;203:489–97.
Finkelstein SD, Sayegh R, Christensen S, et al. Genotypic classification of colorectal adenocarcinoma—biologic behavior correlates with KRAS mutation type. Cancer. 1993;71:3827–38.
Hemmingsson E, Udde J, Goodpaster BH, et al. KRAS genotypes and outcome in patients with chemotherapy-refractory metastasic colorectal cancer treated with cetuximab. JAMA. 2011;305:564–5.
Geido E, Sciutto A, Rubagotti A, et al. Combined DNA flow cytometry and sorting with KRAS2 mutation spectrum analysis and the prognosis of human sporadic colorectal cancer. Clin Cytometry. 2002;50:216–24.
Kirk R. In colorectal cancer, not all KRAS mutations are created equal. Nature Nature. 2011;8:1–1.
Guerrero S, Casanova I, Farré L, et al. K-RAS codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6.
Liu X, Jakubowski M, Hunt J. KRAS gene mutation in colorectal cancer is correlated with increased proliferation and spontaneous apoptosis. Am J Clin Pathol. 2011;135:245–52.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. E. Pérez-Ruiz and Dr. A. Rueda belong to REDISSEC.
Rights and permissions
About this article
Cite this article
Pérez-Ruiz, E., Rueda, A., Pereda, T. et al. Involvement of K-RAS mutations and amino acid substitutions in the survival of metastatic colorectal cancer patients. Tumor Biol. 33, 1829–1835 (2012). https://doi.org/10.1007/s13277-012-0442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0442-z