Abstract
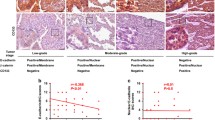
Ezrin, one of the ezrin–radixin–moesin proteins, is involved in the formation of cell membrane processes such as lamellipodia and filopodia and acts as a membrane–cytoskeleton linker. Its aberrant expression correlates with development and progression of several human cancers. However, the expression of ezrin and its role in lung cancer are currently unknown. In this study, we performed ezrin small interfering RNA transfection in two lung cancer cell lines and examined the effects on malignant phenotypes in cancer cells by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, wound healing, and chamber transwell assays. Ezrin knockdown significantly reduced the proliferation, migration, and invasion of lung cancer cells in vitro. To address the possible mechanisms, we evaluated the expression of adhesion molecules E-cadherin and β-catenin by Western blot and reverse transcriptase-polymerase chain reaction analyses. The results demonstrated that downregulation of ezrin reduced β-catenin and increased E-cadherin at the protein level but had no effects on their mRNA levels, suggesting posttranscriptional regulation of these two adhesion molecules. Immunofluorescence assays revealed that ezrin knockdown restored membranous expression of E-cadherin and decreased cytoplasmic β-catenin in lung cancer cells. In addition, ezrin expression was immunohistochemically evaluated on 135 normal and 183 lung cancer tissues. The expression of ezrin was significantly higher in cancer samples than paired autologous normal lung tissues. In normal bronchial epithelium, ezrin was mainly localized on the apical membrane, while in lung cancers and metastatic foci, ezrin was primarily distributed in cytoplasm. Among lung cancer tissues, expression of ezrin was higher in the invasive front of primary lesions and the highest in lymphatic metastasis. Statistical analysis demonstrated that ezrin expression correlated significantly with lymphatic metastasis and advanced TNM stage. Our data suggest that ezrin may play a crucial role in governing the biological behavior of lung cancer.






Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- ERM:
-
Ezrin–radixin–moesin
- ICAM:
-
Intercellular adhesion molecule
- siRNA:
-
Small interfering RNA
- DMSO:
-
Dimethyl sulfoxide
- DAB:
-
Diaminobenzidine
- PVDF:
-
Polyvinylidene fluoride
- HRP:
-
Horseradish peroxidase
- EB:
-
Ethidium bromide
- NSCLC:
-
Non-small cell lung cancer
- FCS:
-
Fetal calf serum
- FITC:
-
Fluorescein isothiocyanate
References
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12.
Shibue T, Weinberg RA. Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin Cancer Biol. 2011;21:99–106.
Lallemand D, Arpin M. Moesin/ezrin: a specific role in cell metastasis? Pigm Cell Melanoma R. 2010;23:6–7.
Neisch AL, Fehon RG. Ezrin, Radixin and Moesin: key regulators of membrane–cortex interactions and signaling. Curr Opin Cell Biol. 2011;23:377–82.
Huang HY, Li CF, Fang FM, Tsai JW, Li SH, Lee YT, et al. Prognostic implication of ezrin overexpression in myxofibrosarcomas. Ann Surg Oncol. 2010;17:3212–9.
Xie JJ, Xu LY, Xie YM, Zhang HH, Cai WJ, Zhou F, et al. Roles of ezrin in the growth and invasiveness of esophageal squamous carcinoma cells. Int J Cancer. 2009;124:2549–58.
Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, et al. Ezrin, a membrane–cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002;179:79–86.
Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane–cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–6.
Ana C, Bendahl PO, Åkerman M, Domanski HA, Rydholm A, Engellau J, et al. Ezrin expression predicts local recurrence and development of metastases in soft tissue sarcomas. J Clin Pathol. 2011;64:689–94.
Hunter KW. Ezrin, a key component in tumor metastasis. Trends Mol Med. 2004;10:201–4.
Kocher HM, Sandle J, Mirza TA, Li NF, Hart IR. Ezrin interacts with cortactin to form podosomal rosettes in pancreatic cancer cells. Gut. 2009;58:271–84.
Osawa H, Smith CA, Ra YS, Kongkham P, Rutka JT. The role of the membrane cytoskeleton cross-linker ezrin in medulloblastoma cells. Neuro-Oncology. 2009;11:381–93.
Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. Ezrin, a membrane–cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999;147:31–8.
Martin M, Andreoli C, Sahuquet A, Montcourrier P, Algrain M, Mangeat P. Ezrin Nh2-terminal domain inhibits the cell extension activity of the cooh-terminal domain. J Cell Biol. 1995;128:1081–93.
Li Q, Wu M, Wang H, Xu G, Zhu T, Zhang Y, et al. Ezrin silencing by small hairpin RNA reverses metastatic behaviors of human breast cancer cells. Cancer Lett. 2008;261:55–63.
Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–8.
Pujuguet P, Maestro LD, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–91.
Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–50.
Mackay DJG, Esch F, Furthmayr H, Hall A. Rho-and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. Cell Biol. 1997;138:927–38.
Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science. 2011;331:1553–8.
William NJ, Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol. 2011;8:611–9.
Zhang XQ, Chen GP, Wu T, Yan JP, Zhou JY. Expression and clinical significance of ezrin in non-small-cell lung cancer. Clin Lung Cancer. 2011 Dec 1. doi:10.1016/j.cllc.2011.04.002.
Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110:3011–8.
Chen L, Hughes RA, Baines AJ, Conboy J, Mohandas N, An X. Protein 4.1R regulates cell adhesion, spreading, migration and motility of mouse keratinocytes by modulating surface expression of beta 1 integrin. J Cell Sci. 2011;124:2478–87.
Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81.
Ren L, Hong SH, Cassavaugh J, Osborne T, Chou AJ, Kim SY, et al. The actin–cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene. 2009;28:792–802.
Estecha A, Sanchez-Martin L, Puig-Kroeger A, Bartolome RA, Teixido J, Samaniego RL, et al. Moesin orchestrates cortical polarity of melanoma tumour cells to initiate 3D invasion. J Cell Sci. 2009;122:3492–501.
Tynninen O, Carpen O, Jaaskelainen J, Paavonen T, Paetau A. Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol Appl Neuro. 2004;30:472–7.
Takeichi M, Watabe M, Shibamoto S, Ito F, Oda H, Uemura T, et al. Dynamic control of cell–cell adhesion for multicellular organization. C R Acad Sci III. 1993;316:818–21.
Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin–alpha catenin fusion molecules. J Biol Chem. 1994;127:235–45.
Federici C, Brambilla D, Lozupone F, Matarrese P, de Milito A, Lugini L, et al. Pleiotropic function of ezrin in human metastatic melanomas. Int J Cancer. 2009;124:2804–12.
Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006;66:3629–38.
Brambilla D, Zamboni S, Federici C, Lugini L, Lozupone F, Milito AD, Cecchetti S, Cianfriglia M, Fais S. P-glycoprotein binds to ezrin at amino acid residues 149-242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int J Cancer. 2011, doi: 10.1002/ijc.26285.
Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, et al. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 2000;19:5123–34.
Fais S. Moulding the shape of a metastatic cell. Leuk Res. 2010;34:843–7.
Brambilla D, Fais S. The Janus-faced role of ezrin in “linking” cells to either normal or metastatic phenotype. Int J Cancer. 2009;125:2239–45.
Financial support
This study received financial support from the National Natural Science Foundation of China (grant no. 30700806 to Q-C Li).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Gao, H., Xu, H. et al. Expression of ezrin correlates with malignant phenotype of lung cancer, and in vitro knockdown of ezrin reverses the aggressive biological behavior of lung cancer cells. Tumor Biol. 33, 1493–1504 (2012). https://doi.org/10.1007/s13277-012-0400-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0400-9