Abstract
Bone marrow metastases are formed in the late phases of prostate cancer disease. Stem cell factor (SCF) and granulocyte colony-stimulating factor (G-CSF) are present in the microenvironment of the bone marrow and play a vital role in cell biology therein. The present study was to investigate the influence of SCF and G-CSF on stem-like properties in prostate cancer cell lines. Upon stimulation with SCF or G-CSF, higher levels of CD117, ABCG2, and CD44 were observed in PC-3 and DU145 cells examined by flow cytometry. Simultaneously, the expressions of Oct3/4 and Nanog were upregulated. Moreover, quantitative real-time PCR verified that the increased Nanog under the stimulations was mostly derived from NANOGP8. In parallel with the increasing expressions of these proteins, higher colony and sphere formation efficiencies were seen in these cells in response to the cytokine stimulations. Furthermore, a synergistic effect of SCF and G-CSF on colony and sphere formations and ABCG2 expression was disclosed. Our results indicate a favorable bone marrow niche for prostate cancer cells where higher levels of cell stemness are maintained at least partly by the cytokines SCF and G-CSF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common male malignancy in the Western world. During the development of the prostate cancer disease, malignant cells may spread to other parts of the body to form more aggressive tumors, particularly the metastatic bone tumor [1]. The bone marrow niche stores a variety of cytokines and growth factors, and thus, it could be present as “seed and soil” for the metastatic cancer cells as has been proposed by Paget [2]. The spreading of prostate cancer cells can take place early in the disease, and some of the metastatic cells could alter their phenotype and hide in the bone marrow with a relatively quiescent state or proliferating at a reduced rate [3]. The recurrence and the metastatic process of prostate cancer are also tightly linked with the biology of a rare subpopulation of undifferentiated cells defined as cancer stem cells (CSCs) within tumors, exhibiting stem cell characteristics [4]. Data indicate that the bone marrow niche could supply an adequate microenvironment for the survival/proliferation of CSCs and maintain their stemness properties [5].
The bone marrow is the major source of hematopoietic cytokines including stem cell factor (SCF) and granulocyte colony-stimulating factor (G-CSF). SCF is an early-acting hematopoietic cytokine that plays a constitutively important role in the proliferation and survival of pluripotent progenitor cells, together with its receptor CD117 [6]. The G-CSF receptor is present on precursor cells of various hematopoietic cells in the bone marrow, and initiates proliferation and differentiation into mature granulocytes, in response to stimulation by G-CSF [7]. SCF has also been identified in the supernatant of some non-hematopoietic tumor cell lines including breast and small cell lung carcinoma cell lines, suggesting cell growth stimulated by autocrine production of SCF in CD117 receptor-bearing tumor cells [8]. The serum level of SCF is significantly increased in lung cancer patients [9]. The data from Wiesner et al. show that prostate cancer cells could release SCF to the extracellular milieu and also indicate that the SCF–CD117 signaling system has a potential contribution to prostate cancer bone metastasis [10]. Presence of G-CSF and its receptor has been demonstrated in cancer cells such as head and neck, bladder, and ovarian cancer cells and has been associated with the growth of these tumor cells [11–13]. G-CSF productions by lung [14] and bladder [15] cancer cells have been reported to be associated with an invasive behavior of these cancer cells. Matsuoka et al. reported a case of prostate cancer cells to produce G-SCF by immunohistochemical analyses, indicating its prognostic implications in prostate cancer outcome [16].
The combination of SCF and G-CSF has been demonstrated to have a synergistic effect on the increase of proliferation, differentiation, and survival of hematopoietic cells [17], and they are crucial factors in long-term culture of human primitive hematopoietic cells in vitro [18]. However, the combinational influence of SCF and G-CSF on prostate cancer cells has not been fully elucidated. Therefore, we investigated the effect of SCF and G-CSF on the stem-like properties in the prostate cancer cell lines PC-3 and DU145 and also studied whether a synergistic effect of these cytokines could be discovered. In this study, we found that either SCF or G-CSF could upregulate the expressions of stemness-related factors including CD117, ABCG2, CD44, Oct3/4, and Nanog. Even higher levels of CD117, ABCG2, and Oct3/4 were observed in the cells stimulated by combinational application of SCF and G-CSF. Furthermore, the upregulation of Nanog upon stimulation by these cytokines was proved to be mainly derived from the NANOGP8. In addition, SCF and G-CSF could significantly, synergistically increase the capacities of colony formation and sphere formation in these cells.
Materials and methods
Cell culture and treatment
Human prostate cancer cell lines PC-3 and DU145 were purchased from American Type Culture Collection (USA). Cells were maintained in an RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin at 37°C in a humidified incubator of 5% CO2. Recombinant human SCF and recombinant human G-CSF were purchased from Invitrogen. The media were removed and replaced with Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Invitrogen) media overnight prior to treatment with/without SCF (100 ng/mL), G-CSF (10 ng/mL), or in combination for further analyses in both cell lines, according to the previous study [19].
Flow cytometry analyses
The PC-3 and DU145 cells were starved in a serum-free DMEM/F-12 medium overnight and then added with SCF, G-CSF, or a combination of these two cytokines for 24 or 48 h before the surface markers CD117, ABCG2, and CD44 expressions were evaluated by flow cytometry. Anti-CD117 monoclonal antibody directly conjugated with phycoerythrin (PE), anti-ABCG2 monoclonal antibody directly conjugated with PE, and anti-CD44 monoclonal antibody directly conjugated with allophycocyanin (APC) were obtained from BD Pharmingen Company. The antibodies were used at optimized dilutions, and cells were incubated for 30 min on ice in the dark. PE Mouse IgG2b (BD Pharmingen) and APC Mouse IgG2b (BD Pharmingen) isotype controls were used as negative controls. Viable and single cells were gated for each sample and analyzed on a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Immunoblotting
After a 48-h treatment with SCF, G-CSF, or in combination for cells, whole cell extracts were dissolved by a RIPA buffer (25 mM Tris-HCl pH 7.6, 100 mM NaCl, 1% NP40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS); Thermo Scientific Pierce, Germany), with protease inhibitors (0.1 μM aprotinin, 1.0 mM PMSF, 1 μM leupeptin, 1 μM pepstatin) added immediately before use. After centrifugation at 15,000 rpm for 15 min at 4°C, total protein concentrations were measured with a Bio-Rad protein assay according to the manufacturer’s instruction. The samples were heated with a benchtop heater (Model 111002, Boekel Scientific, USA) at 100°C for 10 min in a SDS-loading buffer (500 mM Tris-HCl pH 6.8, 10% glycerol, 2% SDS, 0.6 M DTT, 0.05% bromophenol blue). Then, an equal amount of protein per sample was separated by SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride transfer membrane (BIO-RAD, USA), and blocked with 5% nonfat dry milk in Tris-buffered saline–Tween (TBST) for 2 h at room temperature. The primary antibodies diluted in TBST/5% milk were incubated overnight at 4°C before further secondary antibody incubation. The optimized antibody concentrations used in this study are: Oct3/4, MAB1759, 1 μg/mL; Nanog, AF1997, 1 μg/mL; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), AF5718, 0.2 μg/mL. All the antibodies were purchased from R&D. The corresponding secondary HRP-conjugated antibodies were incubated for 1 h at room temperature, and immunocomplexes were visualized using enhanced chemiluminescence (GE Healthcare, UK).
Quantitative real-time PCR
Total RNA of cells was extracted using the RNeasy Kit (Qiagen, CA, USA) according to the manufacturer's instruction. RNA sample concentrations were quantified using a spectrophotometer (Nanodrop ND-1000, USA) at OD 260/280. Complementary DNA was subsequently synthesized from 5 μg total RNA using the Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA, USA). The conditions for reverse transcription were: 25°C for 10 min, 37°C for 12 min, 85°C for 5 min, followed by holding at 4°C. The mRNA expressions of NANOG1, NANOGP8, and GAPDH were measured by quantitative real-time PCR using a Taqman ABI 7900 Sequence Detector System (Applied Biosystems) according to the published literature [20]. The primers and probes for the detection of NANOG1 and NANOGP8 are the following:
-
NANOG1: forward primer-5′-CGCCCTGCCTAGAAAAGACATTT-3′,
-
NANOG1: reverse primer-5′-AGAAGCCGTCTCTGGCTATAGATAA-3′,
-
NANOG1: probe-CTGCTAAGGACAACATTGAT;
-
NANOGP8: forward primer-5′-CGCCCTGCCTAGAAAAGACATTT-3′,
-
NANOGP8: reverse primer-5′-ACGAGTTTGGATATCTTTAGGGTTTAGAATC-3′,
-
NANOGP8: probe-CCTTGGCTGCCGTCTCTG.
All the primers and probes labeled with FAM-MGB were obtained from Applied Biosystems. The GAPDH quantitative RT-PCR kit (4352934E, Applied Biosystems) was used as an internal control, and the Ct values of the cells without cytokine treatment were used as calibrators for evaluating NANOG1 and NANOGP8 expression levels in response to cytokine stimulation.
Colony formation assay
Single cells (400 cells/well) were planted in six-well plates for overnight incubation to allow for cell attachment. The media were replaced with DMEM/F-12 (Invitrogen) for 24 h before SCF (100 ng/mL), G-CSF (10 ng/mL), or both of these cytokines was added in the culture for 10 days. The cells were fixed with 4% buffered formalin for 15 min and then stained with 1% crystal violet for 30 min. The plates were gently washed with PBS and dried before microscopic colony evaluation. Cell cluster with more than 30 cells was considered as a colony. Colony formation efficiency was evaluated as follows:
Sphere formation assay
The sphere formation assay was performed based on the previously described method [21]. Single cells were seeded at a density of 500 cells/well in ultralow attachment six-well plates (Ultralow Cluster Plates, Life Sciences). Cells were cultivated in serum-free DMEM/F12 media (Invitrogen) with/without SCF (100 ng/mL), G-CSF (10 ng/mL), or in combination. More than 30 cells within a sphere was considered to be a full sphere and counted under inverse microscopy. Sphere formation efficiency was evaluated as follows:
Statistical analyses
All the experiments were performed at least three times. Statistical analyses were performed using Student’s t test (P < 0.05 was considered a statistical significance). Data are shown as mean ± SD, and SPSS software (version 16.0) was used for data analysis.
Results
Synergistic effect of SCF and G-CSF on stem-like phenotype
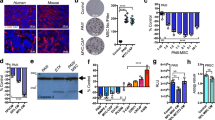
CD117, ABCG2, and CD44 have been described as prostate CSC markers based on clinical investigations and in vitro studies of prostate cancer cell lines [22–24]. Therefore, these surface markers were evaluated by flow cytometry in the PC-3 and DU145 cells with/without SCF or/and G-CSF treatment. As shown in Fig. 1a, CD117 was expressed in both cell lines, and its expression level was significantly upregulated by SCF. Compared to the blank controls, 24 h of stimulation with these cytokines resulted in the following upregulation rates for CD117 expression: there were 4.3-fold, 1.6-fold, and 4.9-fold increases in the PC-3 cells stimulated by SCF, G-CSF, and in combination, respectively; there were 9.1-fold, 5.7-fold, and 10.7-fold increases in the DU145 cells stimulated by SCF, G-CSF, and in combination, respectively (Fig. 1b). After 48 h of stimulation, the upregulation rates for the CD117 expression were as follows: 5.1-fold with SCF, 2.8-fold with G-CSF, and 5.2-fold increases with both cytokines in the PC-3 cells; 18.5-fold with SCF, 16.6-fold with G-CSF, and 21.7-fold increases with both cytokines in the DU145 cells, indicating a time-dependent induction of the CD117 expression in these cells (Fig. 1b).
CD117 expression by flow cytometry. a Representative images of CD117 expressions by flow cytometry in PC-3 and DU145 cell lines by stimulation with cytokines (SCF, G-CSF, or both cytokines for a 24- or 48-h cultivation). Mouse nonimmune IgG2b labeled with PE was used as isotype control corresponding to the antibody of CD117 conjugated with PE (top panel). b Histograms (left panels) and the corresponding tables (right panels) show increasing CD117 expressions in the cells treated with the cytokines for both cell lines. (Asterisk means P < 0.05 vs. blank control; double asterisks means P < 0.01 vs. blank control)
Higher levels of ABCG2 expression were identified in the PC-3 cells cultivated with either SCF (1.7-fold increase for 24 h and 2.3-fold increase for 48 h) or G-CSF (1.6-fold and 1.7-fold increases for the cells in 24- and 48-h cultivations, respectively) (Fig. 2a, b). An even higher expression of ABCG2 was observed in the cells treated by combinational application of these two cytokines: 3.4-fold and 3.5-fold increases for the cells in 24- and 48-h cultivations, respectively (Fig. 2b). Similarly, a significant increase of ABCG2 expression upon stimulation with these cytokines was also observed in the DU145 cells (Fig. 2a). SCF treatment resulted in 21.9-fold and 22.2-fold increases for the cells when cultivated for 24 and 48 h, respectively. G-CSF treatment demonstrated 23.7-fold and 24.0-fold increases for the cells in 24- and 48-h cultivations, respectively. Combinational application of these two cytokines resulted in 32.2-fold and 34.6-fold increases for the cells in 24- and 48-h cultivations, respectively (Fig. 2b), suggesting a synergistic effect of these two cytokines on the ABCG2 expression in these cell lines.
ABCG2 expression by flow cytometry. a Representative images of ABCG2 expressions by flow cytometry in both cell lines with the same treatments as described above. Mouse nonimmune IgG2b labeled with PE was used as isotype control corresponding to the antibody of ABCG2 conjugated with PE. b Histograms (left panels) and the corresponding tables (right panels) show higher expressions of ABCG2 in both cell lines stimulated with either SCF or G-CSF and even higher expressions in the cells treated by combinational application of these cytokines. (Asterisk means P < 0.05 vs. blank control; double asterisks means P < 0.01 vs. blank control)
Figure 3a shows about 50% CD44 positivity in the PC-3 cells and 55% CD44 positivity in the DU145 cells. Comparatively, the CD44 expression was upregulated at higher levels after cytokine stimulation. Application of SCF in the PC-3 cells could result in a 1.6-fold increase of CD44 expression for the cells either in a 24- or 48-h cultivation, and use of SCF in the DU145 cells resulted in a 1.5-fold increase of CD44 expression for the cells in either a 24- or 48-h cultivation. There were 1.5-fold and 1.6-fold increases in CD44 expression in the cells cultivated with G-CSF for 24 and 48 h, respectively (Fig. 3b). Combinational application of both SCF and G-CSF resulted in about 1.7-fold increase in CD44 expression in the PC-3 cells cultivated for 24 and 48 h. In DU145 cells, the upregulated CD44 expression was also observed in the cells treated with SCF and G-CSF. There was about 1.4-fold CD44 increase in CD44 expression in the cells treated with either SCF or G-CSF. There was a 1.5-fold increase in CD44 expression in the cells treated with a combinational application of these cytokines (Fig. 3b).
CD44 expression by flow cytometry. a Representative images of CD44 expressions by flow cytometry in PC-3 and DU145 cell lines with the same treatments as described above. Mouse nonimmune IgG2b labeled with APC was used as isotype control corresponding to the antibody of CD44 conjugated with APC. b Histograms (left panels) and the corresponding tables (right panels) show increasing CD44 expression by stimulation with SCF, G-CSF, or both cytokines in both cell lines. (Asterisk means P < 0.05 vs. blank control; double asterisks means P < 0.01 vs. blank control)
Effect of SCF and G-CSF on stemness marker expressions
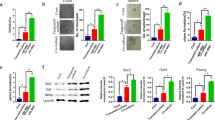
The stemness factors Oct3/4 and Nanog were examined by immunoblotting after cultivation of the PC-3 and DU145 cell lines in the presence or absence of SCF, G-CSF, or both in combination. Oct3/4 and Nanog expressions were upregulated in both cell lines stimulated by SCF, G-CSF, or both cytokines in combination (Fig. 4a). Furthermore, quantitative real-time PCR was used to discriminate whether the upregulated Nanog was derived from NANOG1 or NANOGP8. NANOG1 expression showed almost no response to the stimulation of these cytokines. However, the NANOGP8 expression was dramatically induced by either SCF (2.7-fold increase in PC-3 cells and 2.8-fold increase in DU145 cells) or G-CSF (2.1-fold increase in PC-3 cells and 2.6-fold increase in DU145 cells), and even higher levels of expressions were detected by the combinational treatment of these two cytokines (3.4-fold increase in PC-3 cells and 4.4-fold increase in DU145 cells), which was consistent with the induced Nanog protein expression in both cell lines (Fig. 4b).
Oct3/4 and Nanog expressions by immunoblotting and NANOG1 and NANOGP8 expressions by quantitative real-time PCR. a Immunoblotting analyses show that Oct3/4 and Nanog expressions are increased in the PC-3 and DU145 cell lines treated with SCF, G-CSF, or both cytokines. GAPDH was used as internal loading control. b Either SCF or G-CSF results in a higher NANOGP8 expression, and combinational application of both SCF and G-CSF results in even higher NANOGP8 expression by quantitative real-time PCR. However, NANOG1 shows almost no response to such cytokines
Synergistic effect of SCF and G-CSF on clonogenicity
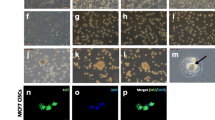
The capacity of clonogenic property was investigated by colony formation and sphere formation assays in these cell lines treated with SCF, G-CSF, or in combination of these cytokines. More colonies were observed in the cells stimulated by either SCF or G-CSF, and even more colonies were seen in the combinational application of these two cytokines (Fig. 5a). Statistical analyses confirmed a significant increase of colony formation efficiency in the cells treated with either SCF or G-CSF and even higher efficiency in the cells treated with both cytokines in both cell lines (Fig. 5b). Both PC-3 and DU145 cells could form spheres at ultralow attachment plates albeit with low efficiency of sphere formation (Fig. 5c). As shown in Fig. 5d, higher sphere formation efficiency was seen in the cells treated with either SCF or G-CSF, and even higher sphere formation efficiency was observed in the cells with the combinational treatment of these two cytokines compared with the blank controls in both cell lines. The results of colony formation and sphere formation suggest a synergistic effect of these two cytokines on the clonogenicity of cells.
Colony formation and sphere formation analyses. a Representative photographs of colony formation show more colonies in the cells treated with either SCF or G-CSF and even more colonies in the cells treated with both cytokines in PC-3 and DU145 cell lines. b Histograms demonstrate higher colony formation efficiency in the cells treated with either SCF or G-CSF and even higher colony formation efficiency in the cells treated with both. c Representative views of sphere formation in PC-3 and DU145 cell lines with/without cytokine treatments. d Histograms show higher sphere formation efficiency in the cells treated with either SCF or G-CSF and even higher sphere formation efficiency in the cells stimulated with both SCF and G-CSF (Asterisk means P < 0.05 vs. blank control; double asterisks means P < 0.01 vs. blank control)
Discussion
Prostate cancer has an affinity to metastasize to the bone marrow, where cytokines like SCF and G-CSF may act on the cell stemness of tumor cells directly or indirectly in autocrine and/or paracrine mechanisms. It is believed that the metastatic cells are prone to be resistant to conventional chemotherapy and radiotherapy, a feature associated with stem-like cell properties in the bone marrow niche. Therefore, we examined how these two cytokines influenced stem-like properties in the prostate cancer cell lines PC-3 and DU145. In our current study, we found that SCF and G-CSF induced the expressions of several surface markers and stemness factors, in addition to the colony and sphere formation potentials in both cell lines, indicating greater stem-like properties of cells in response to SCF and G-CSF.
In accordance with a previous study [25], we found that CD117, the SCF primary receptor, was expressed in prostate cancer cell lines PC-3 and DU145. Increased expressions of SCF and CD117 have been found in several human malignancies including gastrointestinal stromal tumors, acute myeloid leukemia, and gliomas [26]. As expected, our results further showed that the levels of CD117 expression could be induced by SCF stimulation in prostate cancer cells. An increased frequency of CD117 expression in the epithelial compartment of prostate cancer bone metastasis, compared to benign prostatic hyperplasia and primary prostate cancers, has been reported in human specimens by immunohistochemical analyses, suggesting an association between elevated SCF-CD117 signaling and bone metastasis in prostate cancer [10]. SCF, upon binding to its receptor CD117, causes receptor dimerization and activation of tyrosine residues, and signals are transmitted to downstream pathway [27]. The proliferation and invasion of tumor including pancreatic and prostatic cancers could be enhanced through this signaling pathway [10, 28]. In addition, we found that G-CSF also induced subpopulation of CD117 positive cells, whereas the expression level was lower than that induced by SCF or in combinational treatments in these cells, suggesting that SCF plays a major role in the induction of CD117 expression in prostate cancer cells.
The bone marrow is the major source of hematopoietic cytokines including SCF and G-CSF, and this microenvironment is believed to offer protection to the cancer cells in escaping from chemotherapeutic agents. ABCG2 has been widely studied and proven to be associated with multidrug resistance. Since CSCs have been believed to display increasing resistance to conventional therapy, compared to more differentiated tumor cells comprising the majority of mass tumor, the ABCG2 protein is also applied as a marker for the identification of cancer stem-like cells [29]. In our current study, ABCG2 could be significantly induced by either SCF or G-CSF, and these cytokines showed a synergistic effect on the induction of this protein expression in the prostate cancer cell lines. The upregulation of ABCG2 by these cytokines may explain, at least partly, why bone marrow metastatic prostate cancer cells are chemotherapy/radiotherapy-resistant.
The data from Patrawala et al. have shown that CD44+ prostate cancer cells possess inherently greater stem-like characteristics such as being more tumorigenic and metastatic than the corresponding CD44− cells, indicating their association with stem/progenitor cells of prostate cancer [30]. In our present study, we found that CD44 was highly expressed in the PC-3 and DU145 prostate cancer cell lines, and its expression was upregulated by the stimulation of SCF, G-CSF, or in combination of these cytokines. CD44 has been identified as a putative prostate CSC marker, selectively expressed in human prostate cancer cells with neuroendocrine features [31], and such CD44+ cells were suggested to be resistant to hormonal therapy, favoring tumor recurrence following androgen ablation [32, 33]. In line with this finding, the inductive effect of SCF and G-CSF on the expressions of CD117, ABCG2, and CD44 in prostate cancer cells may indicate a role of these cytokines in enriching CSC cells of prostate cancer cells with a feature of drug resistance in a special niche like the bone marrow.
The stemness factors Oct3/4 and Nanog play an important role in maintaining the self-renewal of embryonic stem cells and primordial germ cells. They are also identified in prostate tumor cell lines as well as in primary prostate tumor tissues [34, 35]. In line with previous reports, we found that Oct3/4 and Nanog were expressed in the prostate cancer cell lines PC-3 and DU145. Furthermore, we also found that these stemness factors could be induced by SCF, G-CSF, or combinational stimulation in both cell lines. Sotomayor et al. have found that Oct3/4 expression is overexpressed in prostate cancers with high Gleason scores [35]. It has been reported that Oct3/4 is increasingly expressed in drug-resistant prostate cancer cell lines evaluated by RT-PCR, Western blot, and specific shRNA knock down assays [36]. Nanog has also been reported to be expressed in higher levels in prostate tumors than in the matched benign prostate tissues [37], indicating an important role in maintaining CSC characteristics and prostate cancer resistance to androgen deprivation [20]. Since NANOGP8 expression has been found in cancer [38] and previous studies indicated its important role in maintaining cancer stem-like properties in prostate cancer cells [20, 37], further experiments were performed to distinguish the increased Nanog expression from NANOG1 or NANOGP8. In line with these reports, we repeatedly showed that NANOGP8 was predominantly upregulated by either SCF or G-CSF, and even higher expression was obtained by combinational stimulation of these two cytokines in both cell lines.
SCF and G-CSF are not only available for regulation of growth and differentiation for hematopoietic progenitor cells, but also stimulate the proliferation/invasion of somatic cancer cells such as head and neck carcinoma, pancreatic cancer, colorectal cancer, and bladder and prostatic carcinoma cells [10, 27, 28, 39, 40]. SCF serves as a survival factor to promote cellular proliferation or clonogenic growth in lung cancer [41, 42] and prostate cancer [25]. Moreover, SCF–CD117 autocrine signaling could stimulate proliferation of lung CSCs isolated from human non-small cell lung cancer cell lines in suspension growth [43]. In the present study, SCF also showed a greater induction of colonies and spheres in prostate cancer cells, indicating that the capacity of colony and sphere formations may be associated with the SCF–CD117 axis, based on the upregulation of CD117 by SCF as well. It has been reported that G-CSF could be secreted by PC-3, but not by DU145; however, the receptors of G-CSF were found to be expressed in both PC-3 and DU145 cell lines [44]. In addition, the same report has shown that G-CSF, together with macrophage colony-stimulating factor and IL-3, stimulates the activity of proliferation in the prostate cancer cell line PC-3. Importantly, a similar result was also found in our present study that more colonies and spheres were observed in both cell lines treated with G-CSF alone.
SCF has a synergistic effect with G-CSF for long-term culture of human primitive hematopoietic cells [18] and expansion of human hematopoietic stem/progenitor cells for transplantation in vitro [45, 46]. Similar to these findings, we discovered that there was a synergistic effect of SCF and G-CSF on the induction of colony and sphere formation potentials in prostate cancer cell lines.
Collectively, our data may partly explain why bone marrow metastatic prostate cancer cells are resistant to chemotherapy/radiotherapy, for which much is still unknown in special consideration how metastatic prostate cancer cells in the bone marrow can be dormant for years or decades. Although an increasing potential of sphere and colony formations and high levels of ABCG2 expression influenced by these cytokines may account for their higher stemness in one way, it is still not clear how these cells can be dormant in this niche for a longer period. There must be other important factors for the cells kept dormant in addition to these cytokines in the upregulation of cell stemness. We speculate that at least another factor, TGF-beta, should be explored in order to further characterize the possibility of cell dormancy.
In summary, our results show that SCF and G-CSF could synergistically upregulate the expressions of CSC markers CD117, CD44, and ABCG2, and induce the expressions of transcription factors Oct3/4 and Nanog in prostate cancer cell lines PC-3 and DU145. In parallel with such increasing expressions, there was an increasing potential of colony and sphere formations in these cells, suggesting a role of stemness induction of these cytokines.
References
Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–83.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101.
Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116:1406–18.
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Mroczko B, Szmitkowski M. Hematopoietic cytokines as tumor markers. Clin Chem Lab Med. 2004;42:1347–54.
Knudsen E, Iversen PO, Boyum A, Seierstad T, Nicolaysen G, Benestad HB. G-CSF enhances proliferation and mobilization, but not the maturation rate, of murine myeloid cells. Eur J Haematol. 2011;87:302–11.
Turner AM, Zsebo KM, Martin F, Jacobsen FW, Bennett LG, Broudy VC. Nonhematopoietic tumor cell lines express stem cell factor and display c-kit receptors. Blood. 1992;80:374–81.
Mroczko B, Szmitkowski M, Niklinski J. Stem cell factor and granulocyte-macrophage-colony stimulating factor as candidates for tumour markers for non-small-cell lung cancer. Clin Chem Lab Med. 1999;37:959–62.
Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW, Bittinger F, Thuroff JW, Vessella RL, Cher ML, Bonfil RD. C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia. 2008;10:996–1003.
Sugimoto C, Fujieda S, Sunaga H, Noda I, Tanaka N, Kimura Y, Saito H, Matsukawa S. Granulocyte colony-stimulating factor (G-CSF)-mediated signaling regulates type IV collagenase activity in head and neck cancer cells. Int J Cancer. 2001;93:42–6.
Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J, Nishi T, Amano Y. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995;55:3438–43.
Brandstetter T, Ninci E, Bettendorf H, Perewusnyk G, Stolte J, Herchenbach D, Sellin D, Wagner E, Kochli OR, Bauknecht T. Granulocyte colony-stimulating factor (G-CSF) receptor gene expression of ovarian carcinoma does not correlate with G-CSF caused cell proliferation. Cancer. 2001;91:1372–83.
Pei XH, Nakanishi Y, Takayama K, Bai F, Hara N. Granulocyte, granulocyte-macrophage, and macrophage colony-stimulating factors can stimulate the invasive capacity of human lung cancer cells. Br J Cancer. 1999;79:40–6.
Sawazaki H, Taki Y, Takeuchi H. Granulocyte colony-stimulating factor (G-CSF) producing bladder cancer subsequently developed from recurrent non-muscle invasive bladder cancer. Int J Urol. 2010;17:741–2.
Matsuoka Y, Arai G, Okada Y, Aida J. Prostate cancer-producing granulocyte colony-stimulating factor. Urol Int. 2009;82:113–5.
Duarte RF, Franf DA. The synergy between stem cell factor (SCF) and granulocyte colony-stimulating factor (G-CSF): molecular basis and clinical relevance. Leuk Lymphoma. 2002;43:1179–87.
Nishi N, Ishikawa R, Inoue H, Nishikawa M, Kakeda M, Yoneya T, Tsumura H, Ohashi H, Yamaguchi Y, Motoki K, Sudo T, Mori KJ. Granulocyte-colony stimulating factor and stem cell factor are the crucial factors in long-term culture of human primitive hematopoietic cells supported by a murine stromal cell line. Exp Hematol. 1996;24:1312–21.
Duarte RF, Frank DA. SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood. 2000;96:3422–30.
Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivationJETER2011. Oncogene. 2011;30:3833–45.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70.
Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265–73.
Ugolkov AV, Eisengart LJ, Luan C, Yang XJ. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate. 2011;71:18–25.
Zhang K, Waxman DJ. PC3 prostate tumor-initiating cells with molecular profile FAM65Bhigh/MFI2low/LEF1low increase tumor angiogenesis. Mol Cancer. 2010;9:319.
Savarese DM, Valinski H, Quesenberry P, Savarese T. Expression and function of colony-stimulating factors and their receptors in human prostate carcinoma cell lines. Prostate. 1998;34:80–91.
Gorelik E, Lokshin A, Levina V. Lung cancer stem cells as a target for therapy. Anticancer Agents Med Chem. 2010;10:164–71.
Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H, Manabe T. Stem cell factor/c-kit receptor signaling enhances the proliferation and invasion of colorectal cancer cells through the PI3K/Akt pathway. Dig Dis Sci. 2007;52:2292–300.
Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H, Manabe T. The stem cell factor/c-kit receptor pathway enhances proliferation and invasion of pancreatic cancer cells. Mol Cancer. 2006;5:46.
Pascal LE, Oudes AJ, Petersen TW, Goo YA, Walashek LS, True LD, Liu AY. Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol. 2007;7:6.
Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708.
Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang LS, Yang Q, Huang J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69:787–98.
Huang J, Wu C, di Sant’Agnese PA, Yao JL, Cheng L, Na Y. Function and molecular mechanisms of neuroendocrine cells in prostate cancer. Anal Quant Cytol Histol. 2007;29:128–38.
Amorino GP, Parsons SJ. Neuroendocrine cells in prostate cancer. Crit Rev Eukaryot Gene Expr. 2004;14:287–300.
Bae KM, Su Z, Frye C, McClellan S, Allan RW, Andrejewski JT, Kelley V, Jorgensen M, Steindler DA, Vieweg J, Siemann DW. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. J Urol. 2010;183:2045–53.
Sotomayor P, Godoy A, Smith GJ, Huss WJ. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2009;69:401–10.
Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, Chen H, Chumsri S, Burger AM, Qiu Y. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer. 2010;1:908–16.
Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005.
Zhang J, Wang X, Li M, Han J, Chen B, Wang B, Dai J. NANOGP8 is a retrogene expressed in cancers. FEBS J. 2006;273:1723–30.
Chakraborty A, Guha S. Granulocyte colony-stimulating factor/granulocyte colony-stimulating factor receptor biological axis promotes survival and growth of bladder cancer cells. Urology. 2007;69:1210–5.
Noda I, Fujieda S, Ohtsubo T, Tsuzuki H, Tanaka N, Sunaga H, Saito H. Granulocyte-colony-stimulating factor enhances invasive potential of human head-and-neck-carcinoma cell lines. Int J Cancer. 1999;80:78–84.
Papadimitriou CA, Topp MS, Serve H, Oelmann E, Koenigsmann M, Maurer J, Oberberg D, Reufi B, Thiel E, Berdel WE. Recombinant human stem cell factor does exert minor stimulation of growth in small cell lung cancer and melanoma cell lines. Eur J Cancer. 1995;31A:2371–8.
Sekido Y, Takahashi T, Ueda R, Takahashi M, Suzuki H, Nishida K, Tsukamoto T, Hida T, Shimokata K, Zsebo KM. Recombinant human stem cell factor mediates chemotaxis of small-cell lung cancer cell lines aberrantly expressing the c-kit protooncogene. Cancer Res. 1993;53:1709–14.
Levina V, Marrangoni A, Wang T, Parikh S, Su Y, Herberman R, Lokshin A, Gorelik E. Elimination of human lung cancer stem cells through targeting of the stem cell factor-c-kit autocrine signaling loop. Cancer Res. 2010;70:338–46.
Chackal-Roy M, Niemeyer C, Moore M, Zetter BR. Stimulation of human prostatic carcinoma cell growth by factors present in human bone marrow. J Clin Invest. 1989;84:43–50.
Heike T, Nakahata T. Ex vivo expansion of hematopoietic stem cells by cytokines. Biochim Biophys Acta. 2002;1592:313–21.
Pecora AL. Progress in clinical application of use of progenitor cells expanded with hematopoietic growth factors. Curr Opin Hematol. 2001;8:142–8.
Acknowledgments
We are grateful to Helen Vålerhaugen and Hien D. Dang for their assistance with quantitative real-time PCR, to Idun Dale Rein and Kirsti Solberg Landsverk for their assistance with flow cytometry, and to The Norwegian Radium Hospital Foundation for the financial support.
Conflicts of interest
None
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ma, Y., Liang, D., Liu, J. et al. Synergistic effect of SCF and G-CSF on stem-like properties in prostate cancer cell lines. Tumor Biol. 33, 967–978 (2012). https://doi.org/10.1007/s13277-012-0325-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0325-3