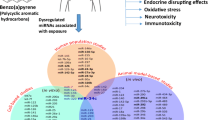
Abstract
We investigated the effect of low-dose, long-term benzo(a)pyrene exposure on the miRNA expression profile in lung tissue of mice, and the potential mechanism of miRNAs in the benzo(a)pyreneinduced damage to health. Subject mice were treated with 5 μg/kg benzo(a)pyrene twice a week for 8 weeks by intragastrical administration, while control mice were treated with the same volume of olive oil solvent. All mice were then fed for another 8 weeks without exposure to benzo(a)pyrene, after which total RNA was isolated from lung tissue. miRNA expression profiles were generated by SOLiD™3 high-throughput sequencing and the signaling pathways represented were analyzed by DIANA-mirPath. A total of 74 miRNAs were dysregulated in mice lung tissues exposed to benzo(a)pyrene. Signaling pathways regulated by benzo(a)pyrene exposure included those involved in the environmental information process and human tumorigenesis. We conclude that low-dose, long-term benzo(a)pyrene exposure alters specific miRNA expression profiles.
Similar content being viewed by others
References
Wogan, G. N., Hecht, S. S., Felton, J. S., Conney, A. H. & Loeb, L. A. Environmental and chemical carcinogenesis. Seminars in Cancer Biology 14:473–486 (2004).
Halappanavar, S. et al. Pulmonary gene and microRNA expression changes in mice exposed to benzo(a)pyrene by oral gavage. Toxicology 285:133–141 (2011).
Croce, C. M. & Calin, G. A. miRNAs, cancer, and stem cell division. Cell 122:6–7 (2005).
Du, T. & Zamore, P. D. MicroPrimer: the biogenesis and function of microRNA. Development 132:4645–4652 (2005).
Skaftnesmo, K. O., Prestegarden, L., Micklem, D. R. & Lorens, J. B. MicroRNAs in tumorigenesis. Curr Pharm Biotechnol 8:320–325 (2007).
Hernando, E. microRNAs and cancer: role in tumorigenesis, patient classification and therapy. Clinical Translational Oncology 9:55–160 (2007).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435:834–838 (2005).
Zhang, B. & Pan, X. RDX induces aberrant expression of microRNAs in mouse brain and liver. Environment Health Perspect 117:231–240 (2009).
Pogribny, I. P. et al. Induction of microRNAome deregulation in rat liver by long-term tamoxifen exposure. Mutat Res 619:30–37 (2007).
Izzotti, A. et al. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 23:806–812 (2009).
Chen, T., Mally, A., Ozden, S. & Chipman, J. K. Low doses of the carcinogen furan alter cell cycle and apoptosis gene expression in rat liver independent of DNA methylation. Environ Health Perspect 118:1597–1602 (2010).
Jardim, M. J., Fry, R. C., Jaspers, I., Dailey, L. & Diaz-Sanchez, D. Disruption of microRNA expression in human airway cells by diesel exhaust particles is linked to tumorigenesis-associated pathways. Environ Health Perspect 117:1745–1751 (2009).
Gaidatzis, D., Van Nimwegen, E., Hausser, J. & Zavolan, M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinformatics 8:69 (2007).
Gusev, Y., Schmittgen, T. D., Lerner, M., Postier, R. & Brackett, D. Computational analysis of biological functions and pathways collectively targeted by COexpressed microRNAs in cancer. BMC Bioinformatics 8:(Suppl7)S16 (2007).
Jemal, A. et al. Cancer statistics. CA Cancer J Clin 59:225–249 (2009).
Johnson, C. D. et al. The let-7 miRNAs represses cell proliferation pathways in human cells. Cancer Res 67: 7713–7722 (2007).
Büssing, I., Slack, F. J. & Grosshans, H. Let-7 micro RNAs in development, stem cells and cancer. Trends Mol Med 14:400–409 (2008).
Takamizawa, J. et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64:3753–3756 (2004).
Boyerinas, B. et al. Identification of let-7-regulated oncofetal genes. Cancer Res 68:2587–2591 (2008).
Ding, X. C., Slack, F. J. & Grosshans, H. The let-7 miRNAs interfaces extensively with the translation machinery to regulate cell differentiation. Cell Cycle 7:3083–3090 (2008).
Huang, Y. et al. Circulating microRNAs as potential biomarkers for smoking-related interstitial fibrosis. Biomarkers 17:435–440 (2012).
Cheung, T. H. et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle 11:2876–2884 (2012).
Wang, X. et al. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod Biomed Online 25:415–424 (2012).
Duan, H., Jiang, Y., Zhang, H. & Wu, Y. MiR-320 and miR-494 affect cell cycles of primary murine bronchial epithelial cells exposed to benzo(a)pyrene. Toxicol In Vitro 24:928–935 (2010).
Chen, J. Y., Wang, C. & Wang, J. MAPK signaling pathway research progress. Chinese Medical Sciences 1:32–34 (2011).
Wang, W., Ren, L. & Wang, J. N. The relationship of MAPK signaling pathway and apoptosis. Chinese Medicine 5:260–261 (2010).
Assoian, R. K., Komoriya, A., Meyers, C. A., Miller, D. M. & Sporn, M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 258:7155–7160 (1983).
Surendran, K., Schiavi, S. & Hruska, K. A. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction and recombinant secreted frizzled related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16:2373–2784 (2005).
Howard, B. & Ashworth, A. Signaling pathways implicated in early mammary gland morphogenesis and breast cancer. PloS Genet 2:e112 (2006).
Heitman, J., Movva, N. R. & Hall, M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Scinece 253:905–909 (1991).
Zheng, P. S. & Ji, J. mTOR signaling pathway with tumor progress. Xi’an Jiaotong University (Medical Sciences) 31:1–9 (2010).
Mascaux, C. et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Cancer 92: 131–139 (2006).
Ludovini, V. et al. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. Journal of Thoracic Oncology 6:707–15 (2011).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 (2007).
Brognard, J., Clark, A. S., Ni, Y. & Dennis, P. A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–3997 (2001).
Zhou, C. et al. The cyclin D1 (CCND1) G870A polymorphism and lung cancer susceptibility: a meta-analysis. Tumour Biol 34:3831–3837 (2013).
Hsia, T. C. et al. Interaction of CCND1 genotype and smoking habit in Taiwan lung cancer patients. Anticancer Res 31:3601–3605 (2011).
Papadopoulos, G. L., Alexiou, P., Maragkakis, M., Reczko, M. & Hatzigeorgiou, A. G. DIANA-mirPath: Integrating human and mouse microRNAs in pathways. Bioinformatic Applications Note 25:1991–1993 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, X., Fu, Y. et al. Expression profiling and pathway analysis of microRNA expression in the lungs of mice exposed to long-term, low-dose benzo(a)pyrene. Mol. Cell. Toxicol. 10, 67–74 (2014). https://doi.org/10.1007/s13273-014-0008-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-014-0008-9