Abstract
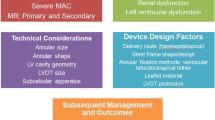
Transcatheter Mitral Valve Replacement (TMVR) is currently under clinical investigation as a viable treatment option for mitral regurgitation (MR). Therefore, it is important to outline the key functional requirements of a TMVR prosthesis in order to provide an overall approach to assessing mitral valve replacement devices utilizing a combination of in vitro and preclinical methods. This article provides a review of the mitral valve disease as well as general considerations and guidance for developing a TMVR device based on International Industry Standards. Specific details pertaining to the mitral valve apparatus, morphology of mitral valve disease, assessment of specific patient population as well as hazard analysis to evaluate and develop a TMVR device to treat a specific patient population have been included. The details contained within this report are not all inclusive or explicate for every technology being developed but rather thought of as a general guide on how a TMVR technology could be developed in alignment with International Industry Standards. Key learnings from the Transcatheter Aortic Valve Replacement (TAVR) experience has also been considered and taken into account when outlining this general guidance for TMVR. Key learning points from the TAVR development experience included the following: quantification of acceptable levels of paravalvular leak, valve migration potential using various anchoring methods and overall implant frame failure modes when treating the native aortic valve. It should be noted that TAVR is over a decade further along in development and clinical experience compared to TMVR. These key learnings from the early experience with TAVR should be considered with all transcatheter development projects.



Similar content being viewed by others
References
Carpentier, A. Cardiac valve surgery—the “French correction”. J. Thorac. Cardiovasc. Surg. 86:323–337, 1983.
Dumesnil, J. G., G. N. Honos, M. Lemieux, and J. Beauchemin. Validation and applications of mitral prosthetic valvular areas calculated by Doppler echocardiography. Am. J. Cardiol. 65:1443–1448, 1990.
Enriquez-Sarano, M., C. W. Akins, and A. Vahanian. Mitral regurgitation. Lancet 373:1382–1394, 2009.
He, Z., and S. Bhattacharya. Mitral valve annulus tension and the mechanism of annular dilation: an in vitro study. J. Heart Valve Dis. 19:701–707, 2010.
Iung, B., G. Baron, P. Tornos, C. Gohlke-Bärwolf, E. G. Butchart, and A. Vahanian. Valvular heart disease in the community: a European experience. Curr. Probl. Cardiol. 32:609–661, 2007.
Jensen, M. O., J. L. Honge, J. A. Benediktsson, A. W. Siefert, H. Jensen, A. P. Yoganathan, T. K. Snow, J. M. Hasenkam, H. Nygaard, and S. L. Nielsen. Septal-lateral mitral valve annular downsizing forces: implications for novel restrictive annuloplasty devices. J. Thoracic Cardiovasc. Surg. 148(1):83–89, 2014.
Jensen, M. O., H. Jensen, M. Smerup, R. A. Levine, A. P. Yoganathan, H. Nygaard, J. M. Hasenkam, and S. L. Nielsen. Saddle-shaped mitral valve annuloplasty rings provide superior annular force distribution compared with flat rings. Circulation 118(suppl 1):250–255, 2008.
Lam, B. K., V. Chan, P. Hendry, M. Ruel, R. Masters, P. Bedard, B. Goldstein, F. Rubens, and T. Mesana. The impact of patient-prosthesis mismatch on late outcomes after mitral valve replacement. J. Thorac. Cardiovasc. Surg. 133:1464–1473, 2007.
Li, M., J. G. Dumesnil, P. Mathieu, and P. Pibarot. Impact of valve prosthesis patient mismatch on pulmonary arterial pressure after mitral valve replacement. J. Am. Coll. Cardiol. 45:1034–1040, 2005.
Magne, J., P. Mathieu, J. G. Dumesnil, D. Tanné, F. Dagenais, D. Doyle, and P. Pibarot. Impact of prosthesis-patient mismatch on survival after mitral valve replacement. Circulation 115:1417–1425, 2007.
Maissano, et al. A translational “humanised” porcine model for transcatheter mitral valve interventions: the neo inferior vena cava approach. EuroIntervention 11:92–95, 2015.
McCarthy, K. P., L. Ring, and B. S. Rana. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur. J. Echocardiogr. 11:i3–i9, 2010.
Nkomo, V. T., J. M. Gardin, T. N. Skelton, J. S. Gottdiener, C. G. Scott, and M. Enriquez-Sarano. Burden of valvular heart diseases: a population-based study. Lancet 368:1005–1011, 2006.
Rausch, M. K., W. Bothe, J. P. Kvitting, J. C. Swanson, D. C. Miller, and E. Kuhl. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann. Biomed. Eng. 40:750–761, 2012.
Siefert, A. W., J. H. Jimenez, K. J. Koomalsingh, F. Aguel, D. S. West, T. Shuto, T. K. Snow, R. C. Gorman, J. H. Gorman, III, and A. P. Yoganathan. Contractile mitral annular forces are reduced with ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2012. https://doi.org/10.1016/j.jtcvs.2012.10.006.
Siefert, et al. Dynamic assessment of mitral annular force profile in an ovine model. Ann. Thorac. Surg. 94(1):59–65, 2012.
Singh, J. P., J. C. Evans, D. Levy, M. G. Larson, L. A. Freed, D. L. Fuller, B. Lehman, and E. J. Benjamin. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (The Framingham Heart Study). Am. J. Cardiol. 83:897–902, 1999.
Stone, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 1: clinical trial design principles. JACC 66(3):278–307, 2015.
Tanné, D., L. Kadem, R. Rieu, and P. Pibarot. Hemodynamic impact of mitral prosthesis-patient mismatch on pulmonary hypertension: an in silico study. J. Appl. Physiol. 295:1916–1926, 2008.
Trichon, B. H., and C. M. O’Connor. Secondary mitral and tricuspid regurgitation accompanying left ventricular systolic dysfunction: is it important, and how is it treated? Am. Heart J. 144:373–376, 2002.
Zoghbi, et al. ASE GUIDELINES AND STANDARDS recommendations for noninvasive evaluation of native valvular regurgitation. J. Am. Soc. Echocardiogr. 30(4):303–371, 2017.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors. This manuscript was created to provide guidance on conducting example studies but no actual data was included within.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Appendix A1: Example—Device Product Development
Appendix A1: Example—Device Product Development
Rights and permissions
About this article
Cite this article
Iyer, R., Chalekian, A., Lane, R. et al. Transcatheter Mitral Valve Replacement: Functional Requirements for Device Design, Bench-Top, and Pre-Clinical Evaluation. Cardiovasc Eng Tech 9, 301–338 (2018). https://doi.org/10.1007/s13239-018-0364-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-018-0364-z