Abstract
Time-varying elastance models (TVEMs) are often used for simulation studies of the cardiovascular system with a left ventricular assist device (LVAD). Because these models are computationally expensive, they cannot be used for long-term simulation studies. In addition, their equilibria are periodic solutions, which prevent the extraction of a linear time-invariant model that could be used e.g. for the design of a physiological controller. In the current paper, we present a new type of model to overcome these problems: the mean-value model (MVM). The MVM captures the behavior of the cardiovascular system by representative mean values that do not change within the cardiac cycle. For this purpose, each time-varying element is manually converted to its mean-value counterpart. We compare the derived MVM to a similar TVEM in two simulation experiments. In both cases, the MVM is able to fully capture the inter-cycle dynamics of the TVEM. We hope that the new MVM will become a useful tool for researchers working on physiological control algorithms. This paper provides a plant model that enables for the first time the use of tools from classical control theory in the field of physiological LVAD control.




Similar content being viewed by others
References
Amacher, R., G. Ochsner, A. Ferreira, S. Vandenberghe, and M. Schmid Daners. A robust reference signal generator for synchronized ventricular assist devices. IEEE Trans. Bio-Med. Eng. 60:2174–2183, 2013.
Batzel, J. J., F. Kappel, D. Schneditz, and H. T. Tran. Cardiovascular and respiratory systems: modeling, analysis, and control.SIAM 34:2007.
Codrean, A. and T.-L. Dragomir. Averaged modeling of the cardiovascular system. In: Proceedings of 52nd IEEE Conference on Decision and Control, 2013, pp. 2066–2071.
Colacino, F. M., F. Moscato, F. Piedimonte, M. Arabia, and G. A. Danieli. Left ventricle load impedance control by apical VAD can help heart recovery and patient perfusion: a numerical study. ASAIO J. 53:263–277, 2007.
Granegger, M., F. Moscato, F. Casas, G. Wieselthaler, and H. Schima. Development of a pump flow estimator for rotary blood pumps to enhance monitoring of ventricular function. Artif. Organs 36:691–699, 2012.
Guzzella, L. and C. Onder. Introduction to Modeling and Control of Internal Combustion Engine Systems. Berlin: Springer, 2009.
Heldt, T., J. Chang, J. Chen, G. Verghese, and R. Mark. Cycle-averaged dynamics of a periodically driven, closed-loop circulation model. Control Eng. Pract. 13:1163–1171, 2005.
Mansouri, M., R. F. Salamonsen, E. Lim, R. Akmeliawati, and N. H. Lovell. Preload-based Starling-like control for rotary blood pumps: numerical comparison with pulsatility control and constant speed operation. PLOS One 10, 2015.
Ochsner, G., R. Amacher, M. J. Wilhelm, S. Vandenberghe, H. Tevaearai, A. Plass, A. Amstutz, V. Falk, and M. Schmid Daners. A physiological controller for turbodynamic ventricular assist devices based on a measurement of the left ventricular volume. Artif. Organs 38:527–583, 2014.
Parlikar, T. A., T. Heldt, and G. C. Verghese. Cycle-averaged models of cardiovascular dynamics. IEEE T. Circuit Syst. 53(11):2459–2468, 2006.
Petrou, A., G. Ochsner, R. Amacher, P. Pergantis, M. Rebholz, M. Meboldt, and M. Schmid Daners. A physiological controller for turbodynamic ventricular assist devices based on systolic left ventricular pressure. Artif. Organs 40(9):842–855, 2016.
Shampine, L. F. and M. W. Reichelt. The MATLAB ODE suite. SIAM J. Sci. Comput. 18:1–22, 1997.
Suga, H. and K. Sagawa. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ. Res. 35(1):117–126, 1974.
Timms, D. A review of clinical ventricular assist devices. Med. Eng. Phys. 33:1041–1047, 2011.
Toy, S. M., J. Melbin, and A. Noordergraaf. reduced models of arterial systems. IEEE T. Bio-Med. Eng. 174–176, 1985.
Tyberg, J. V., J. E. Davies, Z. Wang, W. A. Whitelaw, J. A. Flewitt, N. G. Shrive, D. P. Francis, A. D. Hughes, K. H. Parker, and J.-J. Wang. Wave intensity analysis and the development of the reservoir-wave approach. Med. Biol. Eng. Comput. 47:221–232, 2009.
Vandenberghe, S., P. Segers, P. Steendijk, B. Meyns, R. A. Dion, J. F. Antaki, and P. Verdonck. Modeling ventricular function during cardiac assist: does time-varying elastance work? ASAIO J. 52:4–8, 2006.
Vollkron, M., H. Schima, L. Huber, and G. Wieselthaler. Interaction of the cardiovascular system with an implanted rotary assist device: simulation study with a refined computer model. Artif. Organs. 26:349–359, 2002.
Vollkron, M., H. Schima, L. Huber, R. Benkowski, G. Morello, and G. Wieselthaler. Development of a reliable automatic speed control system for rotary blood pumps. J. Heart Lung Transpl. 24:1878–1885, 2005.
Wang, Y., N. Loghmanpour, S. Vandenberghe, A. Ferreira, B. Keller, J. Gorcsan, and J. Antaki. Simulation of dilated heart failure with continuous flow circulatory support. PloS one. 9:e85234, 2014.
Westerhof, N., J.-W. Lankhaar, and B. E. Westerhof. The arterial windkessel. Med. Biol. Eng. Comput. 47:131–141, 2009.
Acknowledgments
We would like to acknowledge the great support and contribution of Anastasios Petrou to this research. This work is part of the Zurich Heart project under the umbrella of University Medicine Zurich.
Conflict of interest
The authors declare no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Keefe B. Manning and Ajit P. Yoganathan oversaw the review of this article.
Appendix
Appendix
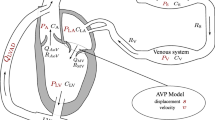
Figure 1a shows the electrical analog of the TVEM. The model has the same three inputs as the MVM (Figure 1b): the pump speed \(N_\mathrm {vad}(t)\), the unstressed volume of the systemic veins \(V_\mathrm {0,sv}(t)\), and the heart rate \(\mathrm {HR}(t)\). The pulmonary and systemic circulations are implemented as described in Section Pulmonary and Systemic Circulations. The heart consists of the right and left ventricles and four valves as described in the following paragraphs.
Table 1 lists all parameters needed to simulate the TVEM and the MVM. The parameter \(f_\mathrm {sys}\) is only used in the MVM and the parameters \(\Phi _1\) and \(\Phi _2\) are only used in the TVEM. All other parameters are used in both models.
Valve Flows
The flows through the mitral and the tricuspid valves are modelled by algebraic equations (no intertances) and are calculated by
where \(p_\mathrm {up}(t)\) and \(p_\mathrm {down}(t)\) are the pressures upstream and downstream of the valve and R is the valve resistance. The flow through the aortic and pulmonary valve \(q_\mathrm {valve}(t)\) is calculated by the differential equation
for the aortic valve flow, \(p_\mathrm {ventricle}(t)\) and \(p_\mathrm {artery}(t)\) are the pressures in the LV and the systemic arteries (aorta), respectively, \(L_\mathrm {valve}\) is the inertance, and \(R_\mathrm {valve}\) is the resistance of the aortic valve, respectively. Similarly, for the pulmonary valve flow, \(p_\mathrm {ventricle}(t)\) and \(p_\mathrm {artery}(t)\) are the pressures in the RV and the pulmonary arteries, respectively, \(L_\mathrm {valve}\) is the inertance, and \(R_\mathrm {valve}\) is the resistance of the pulmonary valve, respectively. The integrator used to solve this differential equation needs to be limited to non-negative values, such that no back flow is possible.
LVAD Flow
The flow through the LVAD \(q_\mathrm {vad}(t)\) is calculated by
where \(k_\mathrm {vad}\), \(R_\mathrm {vad}\), and \(L_\mathrm {vad}\) are the gain, the resistance and the inertance of the LVAD, respectively.
Ventricular Volumes
The RV volume is calculated by the differential equation
where \(q_\mathrm {tv}(t)\) and \(q_\mathrm {pv}(t)\) are the tricuspid and the pulmonary valve flows, respectively. The LV volume is calculated by
where \(q_\mathrm {mv}(t)\) is the mitral valve flow.
Ventricular Pressures
The pressures in both ventricles are calculated by
where \(E_\mathrm {}(t)\) is the time-varying elastance, which is calculated by
The parameters \(E_\mathrm {min}\) and \(E_\mathrm {max}\) are the minimum and maximum elastances, respectively and \(F_{i}(t)\) is the time-varying interpolation function calculated by
where \(\varphi (t)\) describes the cardiac phase calculated by
Rights and permissions
About this article
Cite this article
Ochsner, G., Amacher, R. & Schmid Daners, M. A Novel Mean-Value Model of the Cardiovascular System Including a Left Ventricular Assist Device. Cardiovasc Eng Tech 8, 120–130 (2017). https://doi.org/10.1007/s13239-017-0303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-017-0303-4