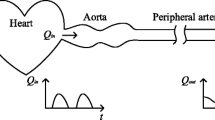
Abstract
To create and characterize a physical Windkessel module that can provide realistic and predictable vascular impedances for in vitro flow experiments used for computational fluid dynamics validation, and other investigations of the cardiovascular system and medical devices. We developed practical design and manufacturing methods for constructing flow resistance and capacitance units. Using these units we assembled a Windkessel impedance module and defined its corresponding analytical model incorporating an inductance to account for fluid momentum. We tested various resistance units and Windkessel modules using a flow system, and compared experimental measurements to analytical predictions of pressure, flow, and impedance. The resistance modules exhibited stable resistance values over wide ranges of flow rates. The resistance value variations of any particular resistor are typically within 5% across the range of flow that it is expected to accommodate under physiologic flow conditions. In the Windkessel impedance modules, the measured flow and pressure waveforms agreed very favorably with the analytical calculations for four different flow conditions used to test each module. The shapes and magnitudes of the impedance modulus and phase agree well between experiment and theoretical values, and also with those measured in vivo in previous studies. The Windkessel impedance module we developed can be used as a practical tool to provide realistic vascular impedance for in vitro cardiovascular studies. Upon proper characterization of the impedance module, its analytical model can accurately predict its measured behavior under different flow conditions.











Similar content being viewed by others
References
Bax, L., C. J. Bakker, W. M. Klein, N. Blanken, J. J. Beutler, and W. P. Mali. Renal blood flow measurements with use of phase-contrast magnetic resonance imaging: normal values and reproducibility. J. Vasc. Interv. Radiol. 16(6):807–814, 2005. doi:16/6/807[pii]10.1097/01.RVI.0000161144.98350.28.
Grant, B. J., and L. J. Paradowski. Characterization of pulmonary arterial input impedance with lumped parameter models. Am. J. Physiol. 252(3 Pt 2):H585–H593, 1987.
Greene, E. R., M. D. Venters, P. S. Avasthi, R. L. Conn, and R. W. Jahnke. Noninvasive characterization of renal artery blood flow. Kidney Int. 20(4):523–529, 1981.
Khunatorn, Y., R. Shandas, C. DeGroff, and S. Mahalingam. Comparison of in vitro velocity measurements in a scaled total cavopulmonary connection with computational predictions. Ann. Biomed. Eng. 31(7):810–822, 2003.
Krenz, G. S., J. H. Linehan, and C. A. Dawson. A fractal continuum model of the pulmonary arterial tree. J. Appl. Physiol. 72(6):2225–2237, 1992.
Ku, J. P., C. J. Elkins, and C. A. Taylor. Comparison of CFD and MRI flow and velocities in an in vitro large artery bypass graft model. Ann. Biomed. Eng. 33(3):257–269, 2005.
Lagana, K., R. Balossino, F. Migliavacca, G. Pennati, E. L. Bove, M. R. de Leval, et al. Multiscale modeling of the cardiovascular system: application to the study of pulmonary and coronary perfusions in the univentricular circulation. J. Biomech. 38(5):1129–1141, 2005. doi:10.1016/j.jbiomech.2004.05.027.
Les, A. S., S. C. Shadden, C. A. Figueroa, J. M. Park, M. M. Tedesco, R. J. Herfkens, et al. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 38(4):1288–1313, 2010. doi:10.1007/s10439-010-9949-x.
Lotz, J., C. Meier, A. Leppert, and M. Galanski. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics 22(3):651–671, 2002.
Segers, P., S. Brimioulle, N. Stergiopulos, N. Westerhof, R. Naeije, M. Maggiorini, et al. Pulmonary arterial compliance in dogs and pigs: the three-element Windkessel model revisited. Am. J. Physiol. 277(2 Pt 2):H725–H731, 1999.
Specht, E. Packomania. In: The Best Known Packings of Equal Circles in the Unit Circle. Germany: University of Magdeburg, 2010, http://www.packomania.com/cci. Accessed 5 May 2010.
Spilker, R. L., J. A. Feinstein, D. W. Parker, V. M. Reddy, and C. A. Taylor. Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann. Biomed. Eng. 35(4):546–559, 2007. doi:10.1007/s10439-006-9240-3.
Steele, B. N., M. S. Olufsen, and C. A. Taylor. Fractal network model for simulating abdominal and lower extremity blood flow during resting and exercise conditions. Comput. Methods Biomech. Biomed. Eng. 10(1):39–51, 2007. doi:770213688[pii]10.1080/10255840601068638.
Stergiopulos, N., B. E. Westerhof, and N. Westerhof. Total arterial inertance as the fourth element of the Windkessel model. Am. J. Physiol. 276(1 Pt 2):H81–H88, 1999.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195(29–32):3776–3796, 2006. doi:10.1016/j.cma.2005.04.014.
Wang, J. J., J. A. Flewitt, N. G. Shrive, K. H. Parker, and J. V. Tyberg. Systemic venous circulation. Waves propagating on a Windkessel: relation of arterial and venous Windkessels to systemic vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 290(1):H154–H162, 2006. doi:00494.2005[pii]10.1152/ajpheart.00494.2005.
Westerhof, N., G. Elzinga, and P. Sipkema. An artificial arterial system for pumping hearts. J. Appl. Physiol. 31(5):776–781, 1971.
Westerhof, N., J. W. Lankhaar, and B. E. Westerhof. The arterial Windkessel. Med. Biol. Eng. Comput. 47(2):131–141, 2009. doi:10.1007/s11517-008-0359-2.
Acknowledgments
The authors would like to thank Lakhbir Johal and Chris Elkins for assistance with the physical construction of the Windkessel modules and with the flow experiments. This work was supported by the National Institutes of Health (grants P50 HL083800, P41 RR09784, and U54 GM072970).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stephen B. Knisley oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kung, E.O., Taylor, C.A. Development of a Physical Windkessel Module to Re-Create In Vivo Vascular Flow Impedance for In Vitro Experiments. Cardiovasc Eng Tech 2, 2–14 (2011). https://doi.org/10.1007/s13239-010-0030-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-010-0030-6