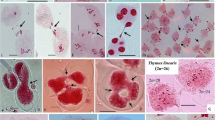
Abstract
We studied meiosis in Mantisia spathulata, a rare, endangered and endemic rhizomatous horticultural herb showing synaptic variation. Most of pollen mother cells (PMCs) analyzed at either diplotene or diakinesis/metaphase I did not exhibit the expected chromosome associations of 10II, which is indicative of synaptic variation. Seventeen percent of the PMCs have shown normal meiotic pattern while 83% PMCs were with abnormal meiotic behavior of bivalents. A total of 419 bivalents and 1,162 of univalents were recorded with significantly low chiasma frequency (8.38 ± 6.20) and terminalization coefficient of 0.82 only. We recorded in 53.33% PMCs, an anomalous distribution pattern of chromosomes, including unequal distribution and/or presence of laggards in the form of univalents/bivalents resulting in low pollen stainability. We suggest that the imbalanced meiosis with variant synaptic behavior of bivalents recorded in M. spathulata may be responsible for loss of genetic variation and considered as substantial cytogenetical factors leading to the rarity and endangeredness of the species.


Similar content being viewed by others
References
Bhowmik SDS, Kumaria S, Tandon P. Conservation of Mantisia spathulata Schult. and Mantisia wengeri Fischer, two critically endangered and endemic zingibers of Northeast India. Seed Tech Notes. 2010;32:57–62.
Bhowmik SSD, Kumaria S, Rao SR, Tandon P. High frequency plantlet regeneration from rhizomatous buds in Mantisia spathulata Schult. and Mantisia wengeri Fischer and analysis of genetic uniformity using RAPD markers. Indian J Exp Biol. 2009;47:140–6.
Cai X, Xu SS. Meiosis-driven genome variation in plants. Curr Genom. 2007;8:151–61.
Dam DP, Dam N, Dutta RM. Mantisia saltatoria Sims versus Globba radicalis Roxb. (Zingiberaceae). Bull Bot Surv India. 1997;34:188–93.
Dhesi JS, Gill BS, Sharma HL. Cytological studies of desynaptic stock in pearl millet, Pennisetum typhoides. Cytologia. 1973;38:311–6.
Elliott EA, Yu J, Ashley T, Arnheim N, Flavell RA, Liskay RM. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995;82:309–19.
Frankham R. Conservation genetics. Annu Rev Genet. 1995;29:305–27.
Frankham R. Genetic management of captive population for reintroduction. In: Serana M, editor. Reintroduction biology of Australian and New Zealand fauna. Chipping Norton, NSW, Australia, Surrey Beatty; 1995. p. 31–34
Ganeshaiah KN. Recovery of endangered and threatened species: developing a national priority list of plant and insects. Curr Sci. 2005;89:599–600.
Hamant O, Ma H, Cande WZ. Genetics of meiotic prophase I in plants. Annu Rev Plant Biol. 2006;57:267–302.
Havekes FWJ, De Jong JH, Heyting C, Ramanna MS. Synapsis and chiasma formation in four meiotic mutants of the tomato Lycopersicon esculentum. Chromosome Res. 1994;2:215–325.
Henderson SA. Temperature and chiasma formation in Schistocera gregaria II. Cytological effects at 40°C and the mechanism of heat induced univalance. Chromosoma. 1962;13:437–63.
John B, Lewis KR. The meiotic system. Protoplasmatologia VI. New York: Springer; 1965.
Kemp B, Boumil RM, Stewart MN, Dawson DS. A role for centromere pairing in meiotic chromosome segregation. Gene Dev. 2004;18:1946–51.
Kitada K, Omura T. Genetic control of meiosis in rice Oryza sativa L. II. Cytogenetical analyses of desynaptic mutants. Jpn J Genet. 1983;58:567–77.
Koykul W, Basrur PK. Synaptic anomalies in fetal bovine oocytes. Genome. 1994;37:83–91.
Kress WJ, Prince LM, Williams KJ. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. Amer J Bot. 2002;89:1682–96.
Kress WJL, Prince M, Hahn WJ, Zimmer EA. Unraveling the evolutionary radiation of the families of the zingiberales using morphological and molecular evidence. Syst Biol. 2001;51:926–44.
Larsen K. Studies on zingiberaceae IV: Caulokaempferia, a new genus. Bot Tidsskr. 1964;60:165–79.
Larsen K, Lock JM, Maas H, Maas PJM. Zingiberaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer; 1998. p. 474–95.
Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya 2: cytogenetics. Notes Roy Bot Gard. 1972;31:229–41.
Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya 3: comparative studies of polyploids. Malays J Sci. 1972;1:19–33.
Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya 4: distribution in relation to polyploidy. Garden’s Bull. 1972;26:115–26.
Lim SN. Cytogenetics and taxonomy of the genus Globba L. (Zingiberaceae) in Malaya 5: introgressive hybridization in hexaploids. Bot J Linn Soc. 1973;66:143–56.
Lim SN. Meiotic instability in the plant genus Globba. In: Wahrman J, Lewis KR, editors. Chromosomes today, 4. New York: Wiley; 1973. p. 321–34.
Maguire MP, Riess RW. Synaptic defects of asynaptic homozygotes in maize at the electron microscope level. Genome. 1996;39:1194–8.
McKently AH, Adams JB. In vitro propagation of Paronychia chartacea. Hort Sci. 1994;29:921–30.
Navarro J, Templado C, Benet J, Lange R, Rajmil O, Egozcue J. Sperm chromosome studies in an infertile man with partial, complete asynapsis of meiotic bivalents. Hum Reprod. 1990;5:227–9.
Newman MF, Jong K. Cytotaxonomic observations on Mantisia wardii (Zingiberaceae). Notes Roy Bot Gard. 1986;43:493–6.
Pandit MK, Babu CR. The effects of loss of sex in clonal populations of an endangered perennial Coptis teeta (Ranunculaceae). Bot J Linn Soc. 2003;143:47–54.
Pazy B, Plitmann U. Asynapsis in cistanche tubulosa (Orobanchceae). Plant Syst Evol. 1996;201:271–3.
Rahman MA, Yusuf M. Mantisia Salarkhanii Rahman & Yusuf (Zingiberaceae)—a new species from Bangladesh. Saudi J Biol Sci. 2002;9:105–10.
Rao SR. Cytogenetics of Phlox and Vigna. Unpublished PhD thesis. India: University of Jodhpur. 1990
Rao SR, Kumar A. Cytological investigations in a synaptic variant of Anogeissus sericea var. sericea Brandis (Combretaceae), an important hardwood tree of Rajasthan. Bot J Linn Soc. 2003;142:103–9.
Sharma SK, Bisht MS, Pandit MK. Synaptic mutation-driven male sterility in Panax sikkimensis Ban. (Araliaceae) from Eastern Himalaya, India. Plant Syst Evol. 2010;287:29–36.
Sharma SK, Bhowmik SSD, Kumaria S, Tandon P, Rao SR. Low genetic variation as revealed by SPAR methods possibly leads to extinction of two critically-endangered and endemic species of Mantisia. Biol Plant. (in press)
Singh DP, Sharma BL, Kanadia BA. Induced variability in mung bean following two methods of handling M2 populations. Trop Grain Legumes Bull. 1980;19:30–4.
Singh RB, Singh BD, Laxmi V, Singh RM. Meiotic behavior of spontaneous and mutagen induced partial desynaptic plants in pearl millet. Cytologia. 1977;42:41–7.
Singh RJ. Plant cytogenetics. London: CRC; 2004.
Sosnikhina SP, Mikhailova EI, Tikholiz OA, Priyatkina SN, Smirnov VG, Voilokov AV, et al. Genetic collection of meiotic mutants of rye Secale cereal L. Russ J Genet. 2005;41:1071–80.
Sudha CG, Seeni S. In vitro propagation of Rauwolfia micrantha, a rare medicinal plant. Plant Cell Tissue Organ. 1996;44:243–8.
Takano A. Cytological analyses of 19 taxa in Globba (Zingiberaceae). Acta Phytaxonomica et Geobotanica. 2001;52:65–74.
Takano A, Okada H. Taxonomy of Globba (Zingiberaceae) in Sumatra, Indonesia. Syst Bot. 2003;28:524–46.
Tandon P, Bhowmik SSD, Mao AA, Kumaria S. Rapid in vitro clonal propagation of Mantisia spathulata Schult, a rare and endemic plant of Northeastern India for recovery. Biotechnology. 2007;6:68–71.
Verma RC, Raina SN. Cytogenetics of Crotalaria VI. Chiasma frequency and position, and univalents behavior in a (partially) asynaptic mutant of C. juncea. Genetica. 1982;58:65–70.
Viera A, Santos JL, Rufas JS. Relationship between incomplete synapsis and chiasma localization. Chromosoma. 2009;118:377–89.
Williams KJ, Kress WJ, Manos PS. The phylogeny, evolution, and classification of the genus Globba and tribe Globbeae (Zingiberaceae): appendages do matter. Am J Bot. 2004;91:100–14.
Xue DW, Ge XJ, Hao G, Zhang CQ. High genetic diversity in a rare, narrowly endemic primrose species: primula interjacens by ISSR analysis. Acta Botanica Sinica. 2004;46:1163–9.
Zetka M, Rose A. The genetics of meiosis in Caenorhabditis elegans. Trends Genet. 1995;11:27–31.
Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754.
Acknowledgements
The present work is supported by a grant from the University Grants Commission, Government of India, New Delhi, through University with Potential for Excellence (UPE)—Bioscience program. Sincere thanks are due to Prof. M.S. Bisht and all members of Plant Biotechnology Laboratories, Department of Botany and Department of Biotechnology and Bioinformatics, NEHU, Shillong, for their constant encouragement and help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S.K., Kumaria, S., Tandon, P. et al. Synaptic variation derived plausible cytogenetical basis of rarity and endangeredness of endemic Mantisia spathulata Schult. Nucleus 54, 85–93 (2011). https://doi.org/10.1007/s13237-011-0033-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-011-0033-1